Molecular interaction of inorganic mercury(ii) with catalase: a spectroscopic study in combination with molecular docking†
Abstract
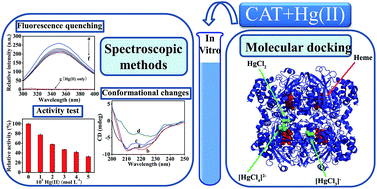
The interaction between inorganic mercury(II) (Hg(II)) and catalase (CAT) was investigated using fluorescence, UV-visible absorption (UV-vis), circular dichroism (CD) spectroscopic techniques and molecular docking methods under simulated physiological conditions (in Tris–HCl buffer, pH = 7.40). The fluorescence quenching analysis showed that the intrinsic fluorescence of CAT was quenched by Hg(II) through a static quenching mechanism. Hg(II) can bind with CAT to form a Hg(II)–CAT complex, with a binding constant of 13.24 L mol−1 at 295 K. Thermodynamic analysis indicated that electrostatic force and van der Waals forces were the dominant intermolecular forces in stabilizing the complex. The results of UV-vis absorption and CD spectral analysis indicated that the formation of the Hg(II)–CAT complex induced some conformational changes in CAT, increasing and decreasing its α-helical content at low and high concentrations of Hg(II), respectively. The CAT activity can be inhibited by Hg(II) significantly, about a 67.2% drop with the presence of 5.0 × 10−4 mol L−1 Hg(II), and the relative activity values of CAT showed a good linear relationship with its fluorescence intensity. Molecular docking was employed to further investigate the interaction of CAT with different species of Hg(II) (HgCl2, [HgCl3]− and [HgCl4]2−), to seek the optimum binding sites of Hg(II) in CAT, and to obtain detailed binding information. This study contributes to the understanding of the interaction mechanism between Hg(II) and CAT at the molecular level in vitro, which is helpful for clarifying the toxicity mechanism of Hg(II) on an antioxidant enzyme system in vivo.

 Please wait while we load your content...
Please wait while we load your content...