Synthesis and characterization of metal-containing poly(siloxane-urethane) crosslinked structures derived from siloxane diols and ferrocene diisocyanate†
Mihaela Dascalu*,
Valentina E. Musteata,
Loredana Vacareanu,
Carmen Racles and
Maria Cazacu
“Petru Poni” Institute of Macromolecular Chemistry, Aleea Gr. Ghica Voda 41 A, Iasi 700487, Romania
First published on 13th November 2015
Abstract
Three new poly(siloxane-urethane) crosslinked structures (PSFUs) were prepared from 1,1′-diisocyanatoferrocene (Fc(NCO)2), and two siloxane diols in different proportions, i.e. α,ω-bis(hydroxybutyl)oligodimethylsiloxane (HS-1) and a hybrid diol containing hydrolysable triethoxy (–Si(–O–Et)3) groups (HS-2). The formation of the urethane groups along the polymer backbone was first confirmed by the presence of the specific bands in FTIR spectra. The resulting materials were characterized using differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), dielectric spectroscopy (DE) and X-ray photoelectron spectroscopy (XPS). Cyclic voltammograms of the compounds showed quasi-reversible oxidation–reduction waves making them suitable for sensing applications.
Introduction
Linear polyurethanes are most commonly formed by reacting a diisocyanate with a diol. A large variety of such precursors could be used to obtain polyurethanes thus offering huge possibilities to adjust their molecular structures according to specific property requirements for certain applications such as elastomers,1 flexible and rigid foams,2 medical devices,3 adhesives and coatings.4 The most commonly used diisocyanates are full organic methylenebis(phenyl isocyanate) (MDI), toluene diisocyanate (TDI), hexamethylene diisocyanate (HDI), naphthalene diisocyanate (NDI), methylene bis-cyclohexylisocyanate (HMDI) (hydrogenated MDI), and isophorone diisocyanate (IPDI). The organic–inorganic diisocynate, 1,1′-diisocyanatoferrocene, can be used as a polyurethane precursor, such an approach allows the insertion of ferrocenyl units within the polyurethane backbone in the context where, there has been an increasing interest in the incorporation of various metals or elements in the main chain of different polymers.5The great development of organometallic polymers field resulted from the fact that the incorporation of transition metals into polymeric structures allows access to special materials with unusual and attractive characteristics. Mainly ferrocenyl-based polymers and copolymers are of high importance due to their redox, electrical, conducting/semiconducting, optical, magnetic, catalytic, preceramic and elastomeric properties.6–8 The broad area of application of the ferrocene polymers includes the manufacture of electronic devices such as microelectrochemical diodes,9 formation of redox gels, which show charge transfer properties,10 modification of electrodes,11 construction of amperometric biosensors,12 precursors to ferromagnetic ceramics,13,14 and more recently, applications in the area of non-linear optical (NLO) materials.15–18
In general, the polyurethanes combine rigid hard and flexible soft segments and, depending on the types and compositions of the two phases as well as the preparation procedures, the relationships between structure and properties of polyurethanes are extremely diverse and could be easily controlled.19–22 The hard segment is formed from diisocyanate moieties, while the soft segment results from the chain extenders that generally are flexible polyols23,24 which contribute to flexibility and elasticity. The number of the diols suitable and used for polyurethanes building is extremely larger mostly being organic ones. However, siloxane diols were also used as reagent for diisocyanate and polyurethane extender.24 The combination between polyurethanes and polysiloxanes, both well known as versatile multipurpose materials has attracted special attention due to a series of advantages: a good thermal stability and high flexibility at low temperatures given by the siloxane part and better mechanical strength and abrasion characteristics contributed by polyurethane.25–27
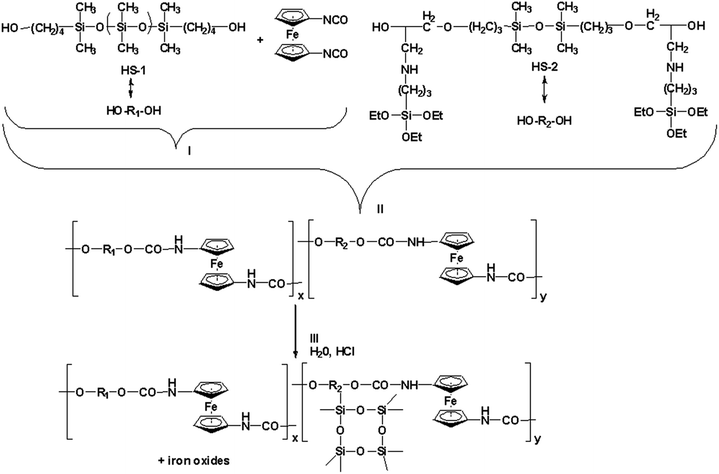
To the best of our knowledge co-existence of ferrocene and siloxane in a polyurethane structure has not been reported to this date, even though, as mentioned above, different combinations of two out of the three main components have been intensively studied. It was the main purpose of our research to obtain combinations of the three main moieties in cross-linked materials and to investigate their properties. Especially the electric properties and electro-chemical behaviour are expected to modify as a result of chemically linked ferrocene. Here we present the synthesis and characterization of new materials based on three main structural elements: ferrocene units and siloxane segments linked by urethane groups. Different from our previous paper,28 where a pure organic monomer was used as urethane precursor, namely isophorone diisocyanate, here we used a less common monomer, 1,1′-diisocyanatoferrocene,29 which permitted the introduction of a metallic element in the form of complexed iron units within the newly formed backbone. Moreover, the resulting poly(siloxane-ferrocene urethane) structures (PSUFs) are crosslinked since a siloxane diol carrying triethoxy groups is used as co-reagent together with an α,ω-bis(hydroxybutyl)oligodimethylsiloxane. The morphology, thermal and dielectric properties of the PSUFs have been investigated. The compounds were analyzed by cyclic voltammetry to evaluate the electrochemical behaviour. In order to determine the precise composition and the oxidation state of the elements present in the resulted materials X-ray photoelectron spectroscopy was used.
Results and discussion
Three new poly(siloxane-urethane) crosslinked structures have been obtained starting with two siloxane diols prepared according to previously reported procedures.28,30 A three-step procedure was applied to prepare the siloxane-urethane crosslinked structures. The first step consisted in the reaction between HS-1 (α,ω-bis(hydroxybutyl)oligodimethylsiloxane) and Fc(NCO)2 (1,1′-diisocyanatoferrocene) in excess, when a prepolymer formed. Then, diol HS-2 (hybrid diol containing hydrolysable triethoxy (–Si(–O–Et)3) groups) was added as chain-extender, in the presence of DBTDL (dibutyltin dilaurate) as catalyst. In the third step H2O and HCl were added, to allow the hydrolysis and condensation of the –Si(–OEt)3 groups leading to the curing of the polymer concomitantly with the formation of an organo-silica network in the case of PSUF3, where an excess of HS2 was used (Scheme 1).The silica domains formed can act as covalent crosslinking points to provide dimensional stability but also act as reinforcement “inorganic filler”. Based on the feeding stoichiometry, the length of the prepolymer differs in PSUF-1 and PSUF-3 (5.25 structural units, theoretical Mn = 5292.11 g mol−1) compared to PSUF-2 (2.77 structural units, theoretical Mn = 2792.22 g mol−1). The lengths of the prepolymers were calculated according to Carothers formula (1) for the case when one of the monomers was used in excess.
 | (1) |
The GPC results on the two prepolymers were in good agreement with the theoretical calculations: Mn,PSUF-3 = 5149 g mol−1, PDI = 1.751; Mn,PSUF-2 = 3124 g mol−1, PDI = 1.801.
Due to the oligomeric nature of HS-1, it acts as the softest part of the poly(siloxane-urethanes), while the length of the prepolymer triggers the chain microstructure, with longer “soft” segments (blocks) in PSUF-1 and 3 and a more “equilibrated” composition in PSUF-2. In addition, as mentioned before, PSUF-3 is reinforced with an organic–inorganic filler generated in situ from the HS-2 used in excess; the presence of such particles is not completely excluded in the other samples.
The FTIR spectra of the PSUFs (Fig. S1-ESI†) showed the disappearance of the band assigned to the isocyanate group at 2295 cm−1,31,32 and the presence of the bands typical for polyurethane: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in urethane group at 1707 cm−1 and –NH (free and bonded) around 3300 cm−1 which is overlapped by the broad band from 3437 cm−1 assigned to OH groups. The hydrolysis of the Si–O–Et groups and their condensation is sustained by the disappearance of the band at 1092 cm−1 assigned to Si–O–C bonds from HS-2 and the appearance of a band around 1198 cm−1 which corresponds to Si–O–Si stretching of crosslinked organo-silica structures resulted by self-condensation of Si-OH groups (formed by hydrolysis of ethoxy groups).33 The band at about 3437 cm−1 and the shoulder at 953 cm−1 are associated with Si-OH groups un-condensed in the described conditions. In the FTIR spectra of the PSUFs (Fig. S1-ESI†), the bands characteristic for the dimethylsiloxane sequences at 1261 and 800 cm−1 are also present. The Si–O–Si band visible in the spectrum of HS-1 at 1020–1080 cm−1 is now overlapped with silica Si–O–Si band and with C–O–C band from the urethane group. The ferrocene sp2 C–H stretching is present at around 3088 cm−1 while asymmetric and symmetric sp3 C–H stretching are assigned at 2963 and 2932 cm−1, respectively.34 The other bands from ferrocene at 1020–1098 cm−1 are overlapped with the Si–O–Si band. The ferrocene Cp–Fe stretching appears at 478 cm−1 in the starting diisocyanate.35 In the PSUFs, a wide band centered at around 460 cm−1 is present. Since in the same region the silica network absorption may overlap,36 we compared the infrared spectra of PSUFs with a previously synthesized poly(siloxane-urethane)28 with similar structure but without ferrocene (Fig. S2-ESI, PU2†). We identified the band for silica at 473 cm−1, which appears as a shoulder in PSUFs, while the band at 460 cm−1 was thus assigned to Cp–Fe bonds in the PSUFs.
O in urethane group at 1707 cm−1 and –NH (free and bonded) around 3300 cm−1 which is overlapped by the broad band from 3437 cm−1 assigned to OH groups. The hydrolysis of the Si–O–Et groups and their condensation is sustained by the disappearance of the band at 1092 cm−1 assigned to Si–O–C bonds from HS-2 and the appearance of a band around 1198 cm−1 which corresponds to Si–O–Si stretching of crosslinked organo-silica structures resulted by self-condensation of Si-OH groups (formed by hydrolysis of ethoxy groups).33 The band at about 3437 cm−1 and the shoulder at 953 cm−1 are associated with Si-OH groups un-condensed in the described conditions. In the FTIR spectra of the PSUFs (Fig. S1-ESI†), the bands characteristic for the dimethylsiloxane sequences at 1261 and 800 cm−1 are also present. The Si–O–Si band visible in the spectrum of HS-1 at 1020–1080 cm−1 is now overlapped with silica Si–O–Si band and with C–O–C band from the urethane group. The ferrocene sp2 C–H stretching is present at around 3088 cm−1 while asymmetric and symmetric sp3 C–H stretching are assigned at 2963 and 2932 cm−1, respectively.34 The other bands from ferrocene at 1020–1098 cm−1 are overlapped with the Si–O–Si band. The ferrocene Cp–Fe stretching appears at 478 cm−1 in the starting diisocyanate.35 In the PSUFs, a wide band centered at around 460 cm−1 is present. Since in the same region the silica network absorption may overlap,36 we compared the infrared spectra of PSUFs with a previously synthesized poly(siloxane-urethane)28 with similar structure but without ferrocene (Fig. S2-ESI, PU2†). We identified the band for silica at 473 cm−1, which appears as a shoulder in PSUFs, while the band at 460 cm−1 was thus assigned to Cp–Fe bonds in the PSUFs.
The results of the thermogravimetric analysis of the PSUFs in the 20–750 °C temperature range, in nitrogen, are shown in Fig. S3-ESI.† The slight weight loss below 250 °C can be assigned to traces of solvents remained after the drying process (DMF especially) or resulted in the condensation of residual silanol groups (water), plus the eventually adsorbed humidity. The weight loss in this step increases with the increasing amount of HS-2 used in the reaction. The main decomposition stage occurs in the 250–400 °C range. The residue amount at 700 °C increases in the order PSUF-3 < PSUF-2 < PSUF-1.
The introduction of siloxane segments into polyurethane structures creates thermodynamically immiscible systems and contributes to the microphase separation of rigid and flexible segments. In our case, the phase separation that exists in poly(siloxane-urethane) crosslinked structures PSUF-1 and 3 is evidenced in the DSC curves (Fig. S4 and S5-ESI†) in which two glass transitions (Tg) are identified: one at around −40 °C (Table 1) which was assigned to the prepolymer containing the oligosiloxane component (“soft” segment) and the second one in the positive temperature range, which represents the relaxation of “hard” segments containing HS-2 disiloxane with network nodes. Both Tg values are higher in PSUF-3 than in PSUF-1 due to the excess HS-2, forming the organo-silica network, which acts as reinforcing filler. A single glass transition was detected for PSUF-2, which has a more balanced microstructure, with smaller prepolymer chain. One possible explanation could be that in PSUF-2 the two main components are better mixed (alternated) and the prepolymer chains do not organize in separate domains. The melting of the siloxane–ferrocenyl–urethane crystalline domains takes place in the 58–66 °C temperature range for all three samples, rising in line with the HS-2 content of the main chain. The crystallization temperatures are in the same order, with a more pronounced supercooling effect in PSUF-1 compared with the PSUF-3. This difference between PSUF-1 and PSUF-3 could be the result of the presence of silica in PSUF-3, which limits the mobility of the macromolecular chains.
| Sample | Tg (°C) (2nd heating) | Tm (°C) (1st heating) | Tc (°C) | |
|---|---|---|---|---|
| PSUF-1 | −43.67 | 9.85 | 57.73 | 16.59 |
| PSUF-2 | — | 10.07 | 66.23 | 58.13 |
| PSUF-3 | −38.97 | 20.78 | 60.11 | 39.11 |
SEM micrographs of the cross-section of the prepared samples processed as films are displayed in Fig. 1. Submicronic domains (roughly between 200 and 1000 nm) are observed in all samples. Sample PSUF-2 has the smoothest aspect in cross-section, with very small regular domains and only few larger particles. This aspect correlates with the presence of a single Tg in DSC. The increased HS-2 feeding amount in PSUF-3 leads to formation of large formations, most probably organo-silica particles agglomerated into the network nodes. The EDX analyses proved the presence of all expected elements (C, O, Si, N and Fe).
The presence of the iron in the materials is expected to induce, among others, electrical or conducting/semiconducting properties, so dielectric measurements were carried by sweeping the frequency from 1 Hz to 1 MHz at constant temperatures taken at 5 °C intervals from −140 °C up to 80 °C, which resulted in an effective heating rate of about 1 °C min−1. In order to have an image of dipolar and charge transport processes, the results were discussed as complex dielectric permittivity ε* = ε′ − iε′′ (where ε′ is the storage component and ε′′ is the loss factor which includes the effects of both dielectric loss and conductivity) as well as the calculated ac conductivity:37 σac = ε0ωε′′, where ε0 is the permittivity of free space and ω = 2πf is the angular frequency. The results revealed that the presence of ferrocene units leads to an increase of dielectric constant from ∼2.8 up to 104,38 when the temperature increases in the range −140–80 °C. The curves evidence very well the frequency dependence of dielectric constant. To evidence the effect of temperature on the complex dielectric permittivity for the PSUFs, the isothermal data are re-plotted in Fig. 2 as a function of temperature at several frequencies. At temperature bellow −50 °C, the dependence of dielectric parameters, ε′ and ε′′, highlights regions which are associated to the mobility of the macromolecular chains. ε′′ presents a small amplitude peak shifting to higher temperatures with increasing frequency, which corresponds to the dipolar relaxation associated with the glass transition of amorphous dimethylsiloxane oligomer. This relaxation has been previously evidenced for siloxane polymers.38 With increasing temperature above −40 °C there is an increase with several orders of magnitude both for ε′ and ε′′, especially for PSUF-2 and 3. The steep increase of ε′ at lower frequencies is caused by increased mobility of charge carriers which leads to space charge polarization phenomena.39 The high value of dielectric loss in the same temperature range is also caused by charge migration that determines the dissipation of the field energy in the material. The frequency dependent shoulder (at about −20 and respectively, 0 °C for PSUF-2 and 3) may come from interfacial polarization when charges are trapped within the interfaces of a material, for example between separated phases, amorphous and crystalline regions or at the external electrode–sample interface.40
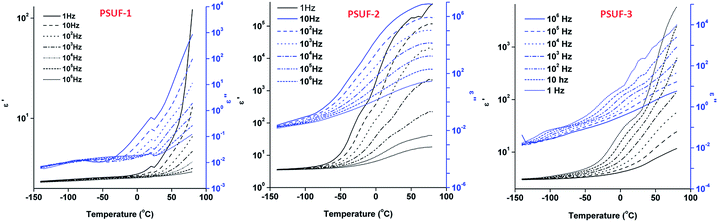 | ||
| Fig. 2 Dielectric permittivity and dielectric loss versus temperature at different frequency (1–10 MHz), for PSUFs. | ||
The frequency dependence of conductivity at several temperatures is shown in Fig. 3. At low temperatures conductivity has close values for all the samples, with a linear dependence on frequency, in double logarithmic scale. The ac conductivity dispersion with increasing frequency is common in heterogeneous and disordered solids41 and corresponds to short range jumps of charges. On increasing temperature, there is a plateau at low frequencies due to charges jumps over long distances which permit electric conduction throughout the volume of the sample.42 The observed plateaus corresponding to dc conductivity σdc at 25 °C are of the order of 10−10 S cm−1 for PUSF-3 and respectively, 10−7 S cm−1 for sample PUSF-2.
For PSUF-1, the frequency independent region appears only at temperatures above 60 °C, and corresponds to lower values of dc conductivity, with σdc ∼ 10−10 S cm−1. At room temperature, the conductivity of PSUFs is several orders of magnitude higher compared to that of polysiloxanes or polyurethanes (∼10−15 to 10−14 S cm−1).43
The Fc(NCO)2 and the PSUFs films were analysed using X-ray Photoelectron Spectroscopy. The Fe high resolution spectra of Fc(NCO)2 revealed a main peak at 708 eV characteristic for Fe(II) in ferrocene and one peak at 709.3 eV which could be assigned to Fe(III) derived from ferrocene (in literature at 709.9 eV).44 This spontaneous oxidation is normal and could occur during manipulation of the sample. In the PSUFs spectra, the region characteristic for Fe exhibited four peaks (two pairs of peaks). The main maximum was detected at approximately 710 eV (PSUF-3 – 710.5 eV, PSUF-2 – 710.1 eV, PSUF-1 – 709.8 eV) with a satellite at approximately 723 eV which was assigned to Fe(III) in ferrocenium moieties. The smaller maximum at approximately 715 eV (715.2 eV-PSUF-3, 714.5 eV-PSUF-2, 715.3 eV-PSUF-1 (Fig. S6†)), with satellites at around 728 eV could be assigned to the presence of iron oxides. According to the literature, for Fe(III) in iron oxides the binding energy is around 711 eV with a satellite around 719 eV.45 The O 1s binding energy in iron oxides was reported to be “nearly invariant at 530.1 eV”.46 In PSUFs, besides the O 1s main peak at approximately 532 eV, there is a small peak at approximately 530 eV (Fig. S6-ESI†). The amount of iron oxides in the three samples could be estimated from the XPS spectra. For example, in the PSUF-3 sample, based on the atomic concentration of O (16.8%) and Fe (3.24%) from the XPS data (not shown), it results that ca. 1.2% O atoms are in iron oxide. Supposing that Fe2O3 is formed, this would mean that ca. 25% from the total ferrocene moieties transformed into iron oxide. The formation of iron oxides is not surprising, given the experimental conditions for the preparation of the crosslinked films, i.e. thermal treatment and the use of HCl.
The electrochemical characteristics of PSUFs were investigated by cyclic voltammetry (CV) in two ways, as free standing film immersed in chloroform in case of PSUF-3 sample, and as chloroform solutions for PSUF-1, PSUF-2 and Fc(NCO)2. All the chloroform solutions contain 0.1 M TBABF4 as supporting electrolyte. Partial oxidation of Fc(NCO)2 due to the manipulation and processing of the sample in normal atmosphere can be the cause for the presence of multiple redox couples in CVs curves, assigned to the Fe(II) as ferrocene and Fe(III) as ferrocenium units (Fig. S7-ESI†). As previously mentioned, the obtained products are slightly flexible crosslinked films, so the preliminary results of the CV measurements that were done for PSUF-3 film immersed in chloroform solution were not satisfactory. This is the reason why the electrochemical measurements for sample PSUF-1 and PSUF-2 where done in solution before they underwent thermal treatment that leads to crosslinked materials and formation of iron oxides. The potential value applied to the working electrode was swept in a large window potential (−1.0 V to 1.0 V), with a 50 mV s−1 scan rate. The cyclic voltammograms recorded for all three samples are shown in Fig. 4.
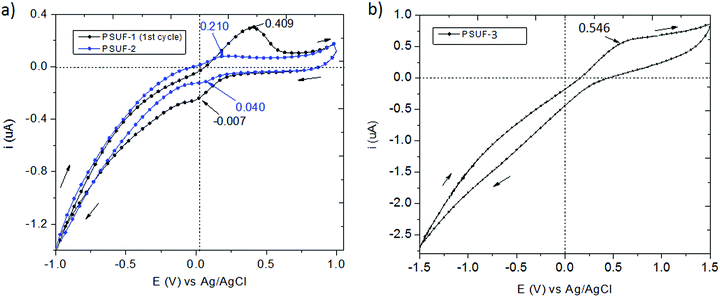 | ||
| Fig. 4 CVs recorded for (a) PSUF-1 and PSUF-2 and (b) PSUF-3 as film in chloroform solutions containing 0.1 M TBABF4 as supporting electrolyte; scan rate of 50 mV s−1. | ||
The recorded CV curves for PSUF-1 and PSUF-2 indicate that the samples undergo one redox process and their electrochemical data are listed in Table 2. The voltammograms indicate one reversible redox process with anodic peaks potential, E (V) of 0.409 V for PSUF-1, and 0.210 V for PSUF-2. The electrons removal in the oxidation step is assumed to ferrocene units and these values of ΔE are 5 and 2 times higher, respectively, compared to ferrocene (Fc) single molecule (ΔE = 0.083 V). The synthesized polymers (PSUF-1 and PSUF-2) show good reversibility of redox processes having half-wave potential at E1/2 = 0.201 V, and 0.125 V vs. Ag/AgCl, respectively. The obtained values are close to those obtained for the reversible redox behaviour of ferrocene-modified polysiloxane (E1/2 = 0.260 V).47,48
| E/V | Sample | ||||
|---|---|---|---|---|---|
| Fc | Fc(NCO)2 | PSUF-1 | PSUF-2 | PSUF-3 | |
| a Epa – anodic peak potential; Epc – cathodic peak potential, ΔE – the peak separation (ΔE = Epa − Epc), E1/2 – the half-wave potential (E1/2 = (Epa + Epc)/2). | |||||
| Epa1 | 0.556 | 0.318 | 0.409 | 0.210 | 0.546 |
| Epc1 | 0.473 | 0.224 | −0.007 | 0.040 | — |
| ΔE1 | 0.083 | 0.094 | 0.416 | 0.170 | — |
| E11/2 | 0.525 | 0.271 | 0.201 | 0.125 | — |
| Epa2 | — | 0.530 | — | — | — |
| Epc2 | — | 0.462 | — | — | — |
| ΔE2 | — | 0.068 | — | — | — |
| E21/2 | — | 0.496 | — | — | — |
| Epa3 | — | 0.800 | — | — | — |
| Epc3 | — | 0.676 | — | — | — |
| ΔE3 | — | 0.124 | — | — | — |
| E31/2 | — | 0.738 | — | — | — |
For PSUF-3 material, the potential was swept from −1.5 V to 1.5 V, and from the voltammogram it can be observed the appearance of only one large and not well-defined oxidation peak (Epa = 0.546 V). Broad shape of the redox peaks can be explained by the slow electron transfer, which is limited by the slow mass transfer of ions through the film upon redox reactions. The current intensity of the cathodic peak is lower than for the anodic peak (and thus difficult to assign precisely) which can be attributed to the slight enhancement of hydrophilic character by the ferrocenium ion state produced upon oxidation.
Materials with fixed redox site on polymer backbone may be used for sensing applications49 or as electrostatic dissipative polymers.50 The route of the redox charge transport may be controlled by either the electron transport within the conducting chain or the electron hopping between redox center and polymer chain.51 From a practical point of view, we recall that a redox group may act as a switching molecular system for charge transport so that interesting applications of redox polymers to electronic devices are open.52 Based on these results, we assume that the un-crosslinked poly(siloxane-urethane)s with ferrocene moieties could be better candidates for sensing elements than the crosslinked materials. On the other hand, a crosslinking reaction in milder conditions could avoid the formation of iron oxides and thus ensure better defined materials for applications.
Experimental
Materials
1,1′-Diisocyanatoferrocene Fc(NCO)2, prepared according to the procedure described in ref. 29 1,3-bis(3-glycidoxypropyl)tetramethyldisiloxane (purity > 95%, b.p. = 184 °C, d420 = 0.996), 3-aminopropyltriethoxysilane (purity > 99%, b.p. = 213–216 °C, d420 = 0.949); dimethyldichlorosilane, (DDS), (purity > 98%, b.p. = 70 °C, d = 1.064), and tetrahydrofurane (THF) were purchased from Fluka; dibutyltin dilaurate (DBTDL), (d420 = 1.055) and magnesium as grains were purchased from Merck; ethyl iodine (d = 1.094) and dimethylformamide (DMF) (purity 99%, b.p. = 152–154 °C, d420 = 0.950, nD20 = 1.430) were purchased from Sigma-Aldrich.Equipments
Fourier transform infrared spectra (FTIR) were recorded on a Bruker Vertex 70 FTIR spectrometer. The analyses were performed in transmission mode, in the 400–4100 cm−1 range, at room temperature with 2 cm−1 resolution and accumulation of 32 scans. The grounded samples were incorporated in dry KBr and processed as pellets. The 1H-NMR spectra were recorded on a BRUKER Avance DRX 400 spectrometer, using CDCl3 as a solvent. The molecular mass of the prepolymers was determined by gel permeation chromatography (GPC) in CHCl3 on a PL-EMD 950 chromatograph/evaporative mass detector instrument. The thermogravimetric analysis was performed on an STA 449F1 Jupiter NETZSCH equipment. The measurements were made in the 20–750 °C temperature range under a nitrogen flow (50 mL min−1) using a heating rate of 10 °C min−1. Alumina crucible was used as sample holder. Differential scanning calorimetry (DSC) measurements were conducted on a DSC 200 F3 Maia (Netzsch, Germany). Nitrogen was used as inert atmosphere at a flow rate of 100 mL min−1. A heating and cooling rate of 10 °C min−1 was applied. Microscopic investigation was performed on an Environmental Scanning Electron Microscope (ESEM) type Quanta 2000 operating at 30 kV with secondary electrons in high vacuum mode. The fractured samples were fixed on Al supports and covered with a thin layer of gold by sputtering (EMITECH K550X). The coupled Energy Dispersive X-ray system (EDX) permitted to perform the qualitative analysis and elemental mapping. The dielectric properties were investigated with a Novoncontrol setup (Broadband dielectric spectrometer Concept 40, Germany), integrating an ALPHA frequency response analyser and a Quatro temperature control system. The measurements were done over a broad frequencies window, 1 to 106 Hz, in the −140–80 °C temperature range. The bias voltage applied across the sample was 1.0 V. Samples having uniform thickness in the 0.2–0.9 mm range were placed between gold plated round electrodes, the upper electrode having a 20 mm diameter. The cyclic voltammetry (CV) measurements were performed on a Bioanalytical System, Potentiostat–Galvanostat (BAS 100B/W). Electrochemical experiments were carried out in a typical three-electrode cell consisting of a glassy carbon electrode (GCE, disk shape, 3.0 mm diameter) as working electrode, a platinum wire as auxiliary electrode, and a reference electrode consisting of a silver wire coated with silver chloride. Cyclic voltammograms of the investigated compounds were recorded in chloroform solution containing tetrabutylammonium tetrafluoroborate (TBABF4) as supporting electrolyte. Prior to each experiment, the prepared solutions were deoxygenated by passing dry argon gas for 10 min. All the electrochemical measurements were carried out in stationary state, at room temperature (25 °C). X-ray photoelectron spectrometry analysis was performed using an Axis Nova spectrometer (Kratos Analytical Ltd.). The pressure in the analysis chamber was typically 3 × 10−9 mbar. The elemental composition was obtained from the survey spectra (pass energy of 160 eV), while high-resolution spectra were collected with a pass energy of 40 eV. All binding energies were referenced to the C 1s peak at 285 eV. The intensities were calculated from the peak areas using Vision Processing software (Vision2 software, Version 2.2.10).Synthesis of siloxane diols
α,ω-Bis(hydroxybutyl)oligo-dimethylsiloxane, HS-1 was obtained following the procedure described in ref. 28 and 30. The oligomer was obtained as viscous oil. The molecular weight of the oligomer (Mn = 740) was estimated by 1H-NMR spectra based on the ratio between the intensity of the peaks assigned to protons from dimethylsiloxane (at 0.08 ppm, 48H) and those from ending butyl units (0.60–3.68 ppm, 16H).The hybrid diol HS-2 was prepared following a previously reported procedure28 starting from 1,3-bis(3-glycidoxypropyl)tetramethyldisiloxane and 3-aminopropyltriethoxysilane in DMF solution using DBTDL as catalyst. Finally a viscous transparent fluid was obtained.
Synthesis of poly(siloxane-urethane) crosslinked structures, PSUFs
For the synthesis of PSUF-1, into a three-neck flask equipped with magnetic stirrer and a nitrogen inlet, HS-1 (1.00 g, 1.35 mmol), Fc(NCO)2 (0.50 g, 1.87 mmol), DMF (15 mL) and DBTDL (0.0084 g) were introduced. After stirring under N2 at 80 °C for 4 h, the temperature was decreased to 70 °C. An appropriate amount of HS-2 solution (0.45 g, 0.60 mmol) was added. The reaction proceeded at 70 °C for another 4 h. The resulted viscous mixture was poured into a large amount of ice-cooled diethyl ether. The precipitation procedure was repeated three times, then H2O and 1.0 M HCl were added in the molar ratio H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl
HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OEt = 2
OEt = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.018
0.018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the solution was stirred at room temperature for 2 h. The resulted dark-brown sol was then poured into a Teflon mold, and kept at 60 °C for 48 h at normal pressure then at 120 °C for another 24 h in a vacuum oven. The other two samples PSUF-2 and PSUF-3 were obtained using the reagents ratios presented in Table 3. By varying the ratio between the two diols, it was expected that the crosslinking degree to increase with the increasing proportion of HS-2 as a result of the introduction of higher number of triethoxysilane groups. All three slightly flexible brown-black films were extracted with chloroform to remove the unreacted compounds/reagents. In PSUF-3, the excess of HS-2 generates organo-silica in situ.
1 and the solution was stirred at room temperature for 2 h. The resulted dark-brown sol was then poured into a Teflon mold, and kept at 60 °C for 48 h at normal pressure then at 120 °C for another 24 h in a vacuum oven. The other two samples PSUF-2 and PSUF-3 were obtained using the reagents ratios presented in Table 3. By varying the ratio between the two diols, it was expected that the crosslinking degree to increase with the increasing proportion of HS-2 as a result of the introduction of higher number of triethoxysilane groups. All three slightly flexible brown-black films were extracted with chloroform to remove the unreacted compounds/reagents. In PSUF-3, the excess of HS-2 generates organo-silica in situ.
| Sample | Reactants | ||
|---|---|---|---|
| HS-1, mmol | HS-2, mmol | Fc(NCO)2, mmol | |
| PSUF-1 | 1.35 | 0.60 | 1.87 |
| PSUF-2 | 1.01 | 1.00 | 1.87 |
| PSUF-3 | 1.35 | 1.60 | 1.87 |
Conclusions
New poly(siloxane-urethane) crosslinked structures were prepared by reacting 1,1′-diisocyanatoferrocene first with an α,ω-bis(hydroxybutyl)oligodimethylsiloxane, followed by the extension of the resulted material with a hybrid siloxane diol containing hydrolysable groups. Due to the insertion of the ferrocene units within the polymeric structures these showed electrochemical activity. As was evidenced by voltammetry measurements, the products undergo one oxidation–reduction process under potential control. The thermogravimetric analysis revealed a greater thermal ability as the feed amount of HS-2 diol increased, while the residue amount increases in the same order. The increasing of the hydrolysable groups content that are silica generators also induces modifications in the morphology of the resulted structures that was emphasized by SEM and DSC. Both ε′ and ε′′ values increase throughout the studied range of temperatures and frequencies following the ratio between HS-2/HS-1. The temperature dependence of both curves show inflections at temperature values close to the transitions detected by DSC. Investigation by XPS suggests the existence of ferrocenium ions and possibly iron oxides formed due to harsh crosslinking reaction conditions.Acknowledgements
The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 264115 – STREAM, and from a grant of the Ministry of National Education, CNCS – UEFISCDI, project number PN-II-ID-PCE-2012-4-0261. We gratefully acknowledge Prof Matthias Tamm from Institut für Anorganische und Analytische Chemie, Technische Universität Braunschweig, Germany for providing us 1,1′-diisocyanatoferrocene, Fc(NCO)2 and for helpful discussion.Notes and references
- C. Hepburn, Polyurethane Elastomers, 2nd edn, Elsevier Science Publisher, New York, 1992 Search PubMed
.
- S. Vlad and S. Oprea, J. Optoelectron. Adv. Mater., 2007, 9, 994–999 CAS
.
- P. Buma, N. N. Ramrattan, T. G. van Tienen and R. P. H. Veth, Biomaterials, 2004, 25, 1523–1532 CrossRef CAS
.
- H. Yeganeh and H. R. Moeini, High Perform. Polym., 2007, 19, 113–126 CrossRef CAS
.
-
(a) H. R. Allcock, Adv. Mater, 1994, 6, 106–115 CrossRef CAS
; (b) I. Manners, Angew. Chem., Int. Ed. Engl., 1996, 35, 1602–1621 CrossRef
.
- I. Manners, Synthetic Metal-Containing Polymers, Wiley-VCH, Weinheim, 2004, p. 130 Search PubMed
.
- S. Fery-Forgues and B. Delavaux-Nicot, J. Photochem. Photobiol., A, 2000, 132, 137–159 CrossRef CAS
.
- B. Knudsen, B. E. Kergl, H. Paulsen, V. Durnev and H. Ritter, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2472–2482 CrossRef CAS
.
- G. P. Kittlesen, H. S. White and H. S. Wrighton, J. Am. Chem. Soc., 1985, 107(25), 7373–7380 CrossRef CAS
.
- H. Z. Bu, A. M. English and S. R. Mikkelsen, J. Phys. Chem. B, 1997, 101(46), 9593–9599 CrossRef CAS
.
- N. C. Foulds and C. R. Lowe, Anal. Chem., 1986, 60(22), 2473–2478 CrossRef
.
- P. D. Hale, L. I. Boguslavsky, T. Inagaki, H. I. Karan, H. S. Lee and T. A. Skotheim, Anal. Chem., 1991, 63(7), 677–682 CrossRef CAS
.
- J. Massey, K. N. Power, I. Manners and M. A. Winnik, J. Am. Chem. Soc., 1998, 120(37), 9533–9540 CrossRef CAS
.
- W. Li, N. Shelter, M. D. Foster, D. Balaishis, I. Manners, B. Annis and J. S. Lin, Polymer, 2000, 41(2), 719–724 CrossRef CAS
.
- H. J. Coles, S. Meyer, P. Lehmann, R. Deschenaux and I. Jauslin, J. Mater. Chem., 1999, 9, 1085–1090 RSC
.
- C. Padeste, A. Grubelnik and L. Tiefenauer, Biosens. Bioelectron., 2000, 15, 431–438 CrossRef CAS PubMed
.
- V. Bennevault-Celton, O. Maciejak, B. Desmazières and H. Cheradame, Polym. Int., 2010, 59, 43–44 CrossRef CAS
.
- M. S. Khan, A. Nigar, M. A. Bashir and Z. Akhter, Synth. Commun., 2007, 37, 473–482 CrossRef CAS
.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS
.
- B. K. Kim, S. Y. Lee and M. Xu, Polymer, 1996, 37, 5781–5793 CrossRef CAS
.
- C. D. Eisenbach and W. Gronski, Makromol. Chem. Rapid Comm., 1983, 4, 707–713 CrossRef CAS
.
- E. Markovic, K. Nguyen, S. Clarke, K. Constantopoulos, J. Matisons and G. P. Simon, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 5038–5045 CrossRef CAS
.
- H. Ulrich, Encyclopedia of Chemical Technology, Wiley-Interscience, New York, 1983, ch. 23, pp. 576–608 Search PubMed
.
- L. Willner, F. Braun, M. Heß and R. Kosfeld, Integration of Fundamental Polymer Science and Technology, ed. P.J. Lemstra and L.A. Kleintjens, Publisher Springer, Netherlands, 1990, part 4, pp. 220–224 Search PubMed
.
- K. Madhavan and B. S. R. Reddy, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2980–2989 CrossRef CAS
.
- R.-S. Chen, C.-J. Chang and Y.-H. Chang, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3482–3490 CrossRef CAS
.
- X. C. Jiang, J. Ding and A. Kumar, J. Membr. Sci., 2008, 323, 371–378 CrossRef CAS
.
- M. Alexandru, M. Cazacu, M. Cristea, A. Nistor, C. V. Grigoras and B. C. Simionescu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49(7), 1708–1718 CrossRef CAS
.
- A. R. Petrov, K. Jess, M. Freytag, P. G. Jones and M. Tamm, Organometallics, 2013, 32(20), 5946–5954 CrossRef CAS
.
- L. Yu and C.-C. Zhang, Iran. Polym. J., 2007, 16(3), 153–160 CAS
.
- E. Pretsch, Tables Spectral Data for Structure Determination of Organic Compounds, 2nd edn, Springer Verlag, Berlin, 1989 Search PubMed
.
- C.-H. Lin, W.-C. Lin and M.-C. Yang, Colloids Surf., B, 2009, 71, 36–44 CrossRef CAS
.
- A. N. Murashkevich, A. S. Lavitskaya, T. I. Barannikova and I. M. Zharskii, J. Appl. Spectrosc., 2008, 75, 730–734 CrossRef CAS
.
- M. Cazacu, A. Vlad, M. Marcu, C. Racles, A. Airinei and G. Munteanu, Macromolecules, 2006, 39, 3786–3793 CrossRef CAS
.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th edn, Wiley, 2009 Search PubMed
.
- L. Tellez, J. Rubio, E. Morales and J. L. Oteo, J. Mater. Sci., 2003, 38, 1773–1780 CrossRef CAS
.
- A. Schonhals and F. Kremer, Broadband Dielectric Spectroscopy, ed. F. Kremer and A. Schönhals, Springer-Verlag, Berlin, 2003, pp. 59–98 Search PubMed
.
- M. Alexandru, M. Cazacu, A. Nistor, V. E. Musteata, I. Stoica, C. Grigoras and B. C. Simionescu, J. Sol-Gel Sci. Technol., 2010, 56, 310–319 CrossRef CAS
.
- K. C. Kao, Dielectric Phenomena in Solids, Elsevier Academic Press, San Diego, USA, 2004, p. 75 Search PubMed
.
- A. Schonhals and F. Kremer, Broadband Dielectric Spectroscopy, ed. F. Kremer and A. Schönhals, Springer-Verlag, Berlin, 2003, pp. 87–93 Search PubMed
.
- G. C. Psarras, Composites, Part A, 2006, 37, 1545–1553 CrossRef
.
- A. K. Jonscher, Universal Relaxation Law, Chelsea Dielectrics Press, London, 1996 Search PubMed
.
-
(a) A. C. M. Kuo, Polymer Data Handbook, ed. J. E. Mark, Oxford University Press, 2nd edn, 2009, ch. 89, pp. 539–561 Search PubMed
; (b) A. S. Hoang, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2011, 2, 025007 CrossRef
.
- R. K. Nagarale, J. M. Lee and W. Shin, Electrochim. Acta, 2009, 54, 6508–6514 CrossRef CAS
.
- A. Vlad, M. Cazacu, C. Turta, R. I. Tigoianu, A. Airinei and A. Arvinte, Synth. Met., 2012, 161, 2659–2668 CrossRef
.
- H.-S. Xu, M.-S. Fang, J.-L. Ju, Q.-D. S. Qin-Chaogu and C.-Z. Yang, Polym. Bull., 1998, 41, 675–679 CrossRef CAS
.
-
(a) T. M. Smith and G. L. Nelson, Polym. Adv. Technol., 2006, 17, 746–753 CrossRef CAS
; (b) G. L. Nelson and N. Naja-Mohajeri, US Pat., 7,041,374 B1, 2006
.
- G. Zotti, S. Zecchin and G. Schiavon, Chem. Mater., 1995, 7, 2309–2315 CrossRef CAS
.
- M. J. Natan and M. S. Wrighton, Prog. Inorg. Chem., 1989, 37, 391–494 CrossRef CAS
.
- G. Riveros, S. Meneses, S. Escobar, C. Garín, B. Chornik and J. Chil, Chem. Soc., 2010, 55(1), 61–66 CAS
.
- T. Fujii, F. M. F. de Groot, G. A. Sawatzky, F. C. Voogt, T. Hibma and K. Okada, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(4), 3195–3202 CrossRef CAS
.
- C. R. Brundle, T. J. Chuang and K. Wandelt, Surf. Sci., 1977, 68, 459–468 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra, TGA and DSC curves, XPS spectra and CV curves of poly(siloxane-urethane) crosslinked structures (PSFUs). See DOI: 10.1039/c5ra15290a |
| This journal is © The Royal Society of Chemistry 2015 |