Photocurrent generation of nanofibers constructed using a complex of a gelator and a fullerene derivative†
Pengchong Xue*,
Panpan Wang,
Boqi Yao,
Jiabao Sun,
Peng Gong,
Zhenqi Zhang and
Ran Lu*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, PR China. E-mail: xuepengchong@jlu.edu.cn; luran@mail.jlu.edu.cn
First published on 1st September 2015
Abstract
A carboxylic acid derivative (C8CBTA) with benzothiadiazole as an electron-withdrawing unit and N-octyl 3-amidocarbazole as an electron donor was designed and synthesized to obtain a gelator with a longer absorption wavelength. C8CBTA could form red gels in some aromatic solvents and could self-assemble into thin nanofibers in gel phases. The changes in UV-vis absorption spectra during gelation suggested J-aggregate stacking between aromatic moieties, and the IR spectrum of the xerogel film revealed the intermolecular hydrogen bonding between amide groups and the formation of dimers between carboxyl groups in gel phases. Thus, the synergistic effect of π–π interaction and hydrogen bonding induced 1D stacking. The C8CBTA gel exhibited a maximal absorption peak at 520 nm and emitted weak near-infrared fluorescence at a maximum of 700 nm because of the strong intramolecular charge transfer. A fullerene derivative that contains an imidazole unit as a hydrogen bond acceptor was synthesized and used as an electron acceptor in constructing a photo-induced electron-transfer two-component gel with C8CBTA. Fullerene derivatives also formed 1D stacking in the two-component gel. Moreover, the xerogel film, which served as the active layer, generated a larger amount of photocurrent compared with the disordered film under light irradiation.
Introduction
Functional organic molecules have been widely developed and applied in numerous practical fields because of their structural flexibility, low cost, and varied properties.1–3 These molecules can self-assemble into nanofibers, nanoribbons, and nanorods, and their size-controlled optical and electrical properties, solution processability, and facile and large-scale synthesis increasingly attract attention.4–6 These 1D nanoscale superstructures are widely used as sensor,7–10 transistor,11,12 batteries,13 solar cell,14 and nanoscale laser.15 Importantly, the gelation of π-gelators in organic solvents or water is a rapid, convenient, and low-cost approach to prepare 1D organic self-assembly.16–22 π–π interaction, which allows long-range exciton migration, exists between π-conjugated moieties in these self-assemblies.23,24 In addition, the narrow diameter of nanofiber provides large surface area. Therefore, the gel nanofibers have been applied in vapor sensors25–28 and transistors.29,30 The π-packing of π-conjugated moieties in the gel nanofibers afforded 1D p- or n-channel; thus, the gel fibers could be used as electron donor or acceptor for photocurrent generation. Shinkai, Würthner, and Stupp et al. developed some gelators as electron donors or acceptors and are applied in preparing physical mixture with gelators and polymers or fullerene derivative for photocurrent generation.31–36 In these systems, p- and n-channels are separated; however, the phase segregation is poor, which is unfavorable for excellent exciton separation.A two-component gel that consists of a donor gelator and a fullerene carboxylic acid derivative as electron acceptor was designed to obtain large-phase segregation and exciton transport channel.36 The hydrogen bonding between the gelator and the fullerene derivative led to 1D close stacking of fullerene derivative. Thus, the electron donor and acceptor were interspersed at the molecular level, and effective electron and hole transport channels existed in the fibers. Consequently, the xerogel fibrous film could generate a large amount of photocurrent under light irradiation. However, a short absorption peak of 357 nm is unfavorable for solar light harvesting. Recently, quinoxaline or 2,3-dimethyl quinoxaline moieties as electron withdrawing group and N-alkyl 3-aminocarbazole or N-alkyl 3-aminocarbazol-3-yl thiophene as electron donor were introduced in the preparation of π-conjugated gelators with a D–π–A–π–D molecular structure to obtain a gelator with longer absorption wavelengths.37,38 Their maximum red-shifted absorption occurred at 487 and 498 nm in their gels. A gelator with longer absorption wavelength was obtained by selecting benzothiadiazole because of its stronger electron-withdrawing ability (Scheme 1). Benzothiadiazole cannot form a strong hydrogen bond with the hydrogen bond donor; thus, a carboxyl terminal group as a hydrogen bond donor was introduced. In addition, a fullerene with imidazole unit as hydrogen bond and electron acceptor was synthesized. As a result, the gelator showed a maximum absorption peak at 520 nm and emitted weak near-infrared fluorescence with a maximum at 700 nm. A hydrogen bonding complex of gelator and fullerene was also formed and self-assembled into 1D nanofibers in the gel phase. Moreover, the xerogel film can provide larger amount of photocurrent compared with the disordered film under light irradiation.
Experimental section
Instruments
Infrared spectra were measured using a Nicolet-360 FT-IR spectrometer by incorporating the samples in KBr disks. The UV-vis spectra were determined on a Mapada UV-1800pc spectrophotometer. C, H, and N elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer. Photoluminescence measurements were taken on a Shimadzu RF-5301 Luminescence Spectrometer. 1H NMR spectra were recorded on Mercury plus 400 MHz and 400 MHz. TEM images were observed with a Hitachi H-8100 apparatus by wiping the samples onto a 200-mesh carbon coated copper grid followed by naturally evaporating the solvent. The fluorescence quantum yields of C8CBTA in THF were measured by comparing to standards (Rhodamine 6G in water, ΦF = 0.75). Cyclic voltammetry was employed using a three-electrode cell and an electrochemistry workstation (CHI 604). The working electrode was a glass carbon disc, the auxiliary electrode was a Pt wire, and Ag/AgCl was used as reference electrode. Tetrabutylammonium tetrafluoroborate (TBABF4, 0.1 M) was used as the supporting electrolyte in dry THF and the ferrocenium/ferrocene (Fc/Fc+) redox couple was used as an internal potential reference.Gelation test of organic fluids
The solution containing certain weighed gelator 1 in organic solvent was heated in a sealed test tube with 1 cm diameter in an oil bath until the solid was dissolved. After the solution was allowed to stand at room temperature for 6 h, the state of the mixture was evaluated by the “stable to inversion of a test tube” method.Preparation of the ITO active electrodes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give light yellow solid (1.5 g, 85% in yield). Element analysis (%): calculated for C22H26N2O2: C, 75.40; H, 7.48; N, 7.99; Found: C, 75.45; H, 7.43; N, 7.94. 1H NMR (400 MHz, CDCl3) δ 9.00 (s, 1H), 8.37 (d, J = 8.8 Hz, 1H), 8.15 (s, 1H), 7.65 (d, J = 8.1 Hz, 1H), 7.39 (t, J = 9.1 Hz, 2H), 7.26 (s, 1H), 6.91 (dd, J = 17.2, 11.0 Hz, 1H), 5.82 (d, J = 17.6 Hz, 1H), 5.28 (d, J = 10.7 Hz, 1H), 4.32 (t, J = 6.4 Hz, 2H), 1.88 (m, 2H), 1.29 (m, 10H), 0.86 (t, J = 7.0 Hz, 3H). MS, m/z: cal. 350.2, found 349.8, M+.
1) to give light yellow solid (1.5 g, 85% in yield). Element analysis (%): calculated for C22H26N2O2: C, 75.40; H, 7.48; N, 7.99; Found: C, 75.45; H, 7.43; N, 7.94. 1H NMR (400 MHz, CDCl3) δ 9.00 (s, 1H), 8.37 (d, J = 8.8 Hz, 1H), 8.15 (s, 1H), 7.65 (d, J = 8.1 Hz, 1H), 7.39 (t, J = 9.1 Hz, 2H), 7.26 (s, 1H), 6.91 (dd, J = 17.2, 11.0 Hz, 1H), 5.82 (d, J = 17.6 Hz, 1H), 5.28 (d, J = 10.7 Hz, 1H), 4.32 (t, J = 6.4 Hz, 2H), 1.88 (m, 2H), 1.29 (m, 10H), 0.86 (t, J = 7.0 Hz, 3H). MS, m/z: cal. 350.2, found 349.8, M+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The mixture was stirred overnight and product as black solid was gained by filtration. Yield: 87%. Element analysis (%): calculated for C50H52N6O4S: C, 72.09; H, 6.29; N, 10.09; Found: C, 72.13; H, 6.21; N, 10.01. 1H NMR (400 MHz, CDCl3) δ 9.04 (s, 2H), 8.41 (m, 4H), 8.23 (d, J = 16.8 Hz, 2H), 7.94 (d, J = 8.5 Hz, 2H), 7.76 (s, 2H), 7.75 (d, J = 16.7 Hz, 2H), 7.51 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 9.1 Hz, 2H), 4.37 (t, J = 7.2 Hz, 4H), 1.56 (m, 4H), 1.49–1.22 (m, 20H), 0.89 (t, J = 7.0 Hz, 6H). MS, m/z: cal. 832.4, found 831.9, M+.
3). The mixture was stirred overnight and product as black solid was gained by filtration. Yield: 87%. Element analysis (%): calculated for C50H52N6O4S: C, 72.09; H, 6.29; N, 10.09; Found: C, 72.13; H, 6.21; N, 10.01. 1H NMR (400 MHz, CDCl3) δ 9.04 (s, 2H), 8.41 (m, 4H), 8.23 (d, J = 16.8 Hz, 2H), 7.94 (d, J = 8.5 Hz, 2H), 7.76 (s, 2H), 7.75 (d, J = 16.7 Hz, 2H), 7.51 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 9.1 Hz, 2H), 4.37 (t, J = 7.2 Hz, 4H), 1.56 (m, 4H), 1.49–1.22 (m, 20H), 0.89 (t, J = 7.0 Hz, 6H). MS, m/z: cal. 832.4, found 831.9, M+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to give light yellow solid (0.45 g, 67% in yield). Element analysis (%): calculated for C50H56N6S: C, 77.68; H, 7.30; N, 10.87; Found: 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 2H), 8.15 (d, J = 16.3 Hz, 2H), 7.78 (d, J = 8.5 Hz, 2H), 7.74 (s, 2H), 7.71 (d, J = 16.4 Hz, 2H), 7.49 (s, 2H), 7.35 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.5 Hz, 2H), 6.93 (d, J = 8.4 Hz, 2H), 4.24 (t, J = 7.0 Hz, 4H), 3.66 (s, 4H), 1.86 (m, 4H), 1.47–1.08 (m, 20H), 0.87 (t, J = 6.5 Hz, 6H).
30) to give light yellow solid (0.45 g, 67% in yield). Element analysis (%): calculated for C50H56N6S: C, 77.68; H, 7.30; N, 10.87; Found: 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 2H), 8.15 (d, J = 16.3 Hz, 2H), 7.78 (d, J = 8.5 Hz, 2H), 7.74 (s, 2H), 7.71 (d, J = 16.4 Hz, 2H), 7.49 (s, 2H), 7.35 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.5 Hz, 2H), 6.93 (d, J = 8.4 Hz, 2H), 4.24 (t, J = 7.0 Hz, 4H), 3.66 (s, 4H), 1.86 (m, 4H), 1.47–1.08 (m, 20H), 0.87 (t, J = 6.5 Hz, 6H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (0.8 g, 28% in yield). Elemental Analysis: C, 69.76; H, 4.68; N, 16.27; found: Elemental Analysis: C, 69.69; H, 4.63; N, 16.32. 1H NMR (500 MHz, CDCl3) δ 10.05 (s, 1H), 8.03 (d, J = 8.6 Hz, 2H), 7.99 (s, 1H), 7.59 (d, J = 8.6 Hz, 2H), 7.38 (s, 1H), 7.27 (s, 1H), 7.27–7.25 (m, 1H).
5) (0.8 g, 28% in yield). Elemental Analysis: C, 69.76; H, 4.68; N, 16.27; found: Elemental Analysis: C, 69.69; H, 4.63; N, 16.32. 1H NMR (500 MHz, CDCl3) δ 10.05 (s, 1H), 8.03 (d, J = 8.6 Hz, 2H), 7.99 (s, 1H), 7.59 (d, J = 8.6 Hz, 2H), 7.38 (s, 1H), 7.27 (s, 1H), 7.27–7.25 (m, 1H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then toluene/ethanol = 20
1 and then toluene/ethanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give black solid (0.1 g, 40% in yield). 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 2H), 7.91 (s, 1H), 7.48 (d, J = 8.4 Hz, 2H), 7.32 (s, 1H), 7.20 (s, 1H), 5.02 (d, J = 9.4 Hz, 1H), 4.99 (s, 1H), 4.30 (d, J = 9.4 Hz, 1H), 2.83 (s, 3H).
1) to give black solid (0.1 g, 40% in yield). 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 2H), 7.91 (s, 1H), 7.48 (d, J = 8.4 Hz, 2H), 7.32 (s, 1H), 7.20 (s, 1H), 5.02 (d, J = 9.4 Hz, 1H), 4.99 (s, 1H), 4.30 (d, J = 9.4 Hz, 1H), 2.83 (s, 3H).Results and discussion
Molecular synthesis
Donor and acceptor units were linked by vinyl group as π-bridge bond because small twist angles between phenyl and vinyl groups would enhance molecular π-conjugated degree and then induce red-shift of absorption spectrum. In addition, an amide moiety and long alkyl chains were introduced to produce a compound that gelates in solvent and self-assembles into 1D nanofibers.41,42 Scheme 2 shows the detailed synthesis route. Compound 3 was synthesized based on reported procedures. A Heck reaction between 4,7-dibromobenzo[c][1,2,5]thiadiazole and compound 3 containing nitro and terminal vinyl moieties produced a high yield (87%) of key intermediate 4. Compound 5 was easily obtained using NH2NH2 as a reducing agent in the presence of Pd/C. The rapid amidation of 4 with succinic anhydride supported the terminal product without purification of the column chromatography. C8CBTA was characterized through elemental analysis, NMR, and MS. C60MZ with imidazole unit as a hydrogen bond acceptor was designed and synthesized because C8CBTA is a hydrogen bond donor. First, compound 6 was obtained through the nucleophilic substitution reaction of imidazole and 4-fluorobenzaldehyde. The reaction between C60, 6, and glycine in o-dichlorobenzene at a refluxing condition for 1 h produced crude C60MZ. Because imidazole unit could bind glycine, the mixture was washed several times with NaOH aqueous solution. Pure C60MZ was obtained through gradient elution column chromatography.Photophysical properties of C8CBTA in solution
The obtained C8CBTA was black in color; thus, a longer absorption wavelength was expected. When C8CBTA dissolved in THF, a red solution was obtained, and the absorption spectrum of THF solution showed absorption peaks at 506, 350, and 319 nm (Fig. 1a and Table 1). The peaks at 350 and 319 nm may have originated from the π–π* transition. The strong absorption at 506 nm exhibited a high molar absorption coefficient (3.05 × 104 M−1 cm−1), which was a longer wavelength compared with that of gelator where quinoxaline served as a relatively weak electron-withdrawing group. This red-shifted absorption suggests that the introduced benzothiadiazole moiety indeed led to a longer absorption wavelength. Fig. 1 shows the solvent-dependent absorption and fluorescence spectra. Increasing the solvent polarity induced a slight red-shift in the absorption band (Table S1†). For example, the maximal absorption peak at 506 nm shifted to 514 nm in DMSO. By contrast, a larger shift of the maximum emission was observed as solvent polarity increased. C8CBTA emitted a strong red fluorescence with a maximum at 620 nm and a fluorescence quantum yield of 0.39. DMSO solution showed an emission peak at 659 nm, indicating a red-shift of 39 nm relative to that in THF; this finding suggests that the excited state exhibited a larger polarity compared with the ground state.43,44 In addition, the shift in the emission band in polar solutions is smaller relative to that in D–π–A molecules because C8CBTA exhibits a symmetric conformation, which was confirmed by quantum chemical calculation. DFT calculation (B3LYP/6-31G) indicated that the maximal absorption band of C8CBTA is assignable to the transition of HOMO to LUMO. Moreover, HOMO is mainly localized over the linear party, whereas LUMO is mainly distributed in the benzothiadiazole and vinyl units (Fig. 1c). Thus, light excitation would lead to a charge transfer from carbazole to benzothiadiazole. However, symmetric C8CBTA resulted in small change in molecular polarity after light excitation; thus, a slight red-shift in fluorescence spectra of C8CBTA was observed.45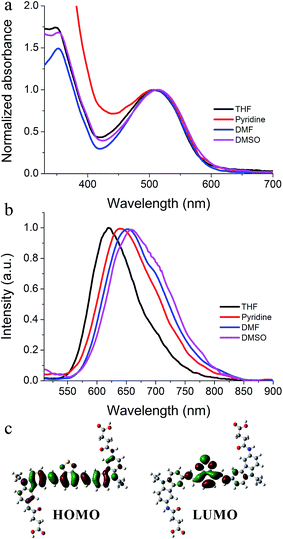 | ||
| Fig. 1 (a) Normalized absorption and (b) fluorescence spectra of C8CBTA in various solvents, and (c) HOMO and LUMO of C2CBTA. | ||
| λabsa (nm, ε × 104 M−1 cm−1) | λema (nm) | Φb | Egc (eV) | HOMOd (eV) | LUMOe (eV) | HOMOf (eV) | LUMOf (eV) | |
|---|---|---|---|---|---|---|---|---|
| a Measured in THF.b Obtained in THF using Rhodamine 6G as reference (0.75 in water, λex = 488 nm).c Determined by the onset of absorption spectrum.d Obtained by first oxidation peak with ferrocene/ferrocenium as an internal reference.e ELUMO = EHOMO − Eg.f Determined after geometrical optimization. | ||||||||
| C8CBTA | 506 (3.05), 350 (4.34), 319 (3.55) | 620 | 0.39 | 2.11 | −4.82 | −2.71 | −4.55 | −2.36 |
Cyclic voltammetry was performed to investigate the electrochemical properties of C8CBTA. The higher HOMO energy level (−4.82 eV) of C8CBTA, which was estimated from the first oxidation potential, is ascribed to the presence of amidocarbazole moiety. The LUMO was evaluated using the following equation: ELUMO = EHOMO + Eg. Eg is the 0–0 excitation energy, which is determined by the onset of absorption spectrum in THF. A LUMO energy level of −2.71 eV was obtained, which is higher relative to that of fullerene derivative. Thus, electron transfer from the gelator to C60MZ is possible, and photocurrent generation is expected.46
Self-assembly in the gel phase
The gelation ability of C8CBTA was first investigated because gelation in organic solvents always involves supramolecular self-assembly and formation of 1D nanofibers in the gel phase. The result is listed in Table 2. C8CBTA is difficult to dissolve in nonpolar hydrocarbon solvents, ethanol, and ethyl acetate even upon heating. Low solubility of C8CBTA was observed in nonpolar aromatic solvents, such as benzene and toluene. By contrast, C8CBTA dissolved in aromatic amines, pyridine, and quindine at room temperature because of the strong interaction between the carboxyl groups of the gelator and the N atoms of the solvents. Clear solutions were observed in polar THF, DMSO, and DMF. Red gels formed when the hot solutions of dichlorobenzene and bromobenzene were cooled to room temperature and aged for 1 h. Low critical gelation concentrations were observed in the two aforementioned solvents. For example, 0.6 mg of C8CBTA could gelate 1 mL of bromobenzene. Thus, one gelator molecule can prevent the flow of more than 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bromobenzene. TEM measurement was performed to observe the morphology of the self-assembly in the gel phase. Fig. 2 shows that the gelators in the gel formed numerous intertwined fibers with a high aspect ratio, indicating that the gelators are likely to stack together to form 1D aggregates.47,48
000 bromobenzene. TEM measurement was performed to observe the morphology of the self-assembly in the gel phase. Fig. 2 shows that the gelators in the gel formed numerous intertwined fibers with a high aspect ratio, indicating that the gelators are likely to stack together to form 1D aggregates.47,48
| Solvent | C8CBTA | C60MZ | Complexc | Solvent | C8CBTA | C60MZ | Complexc |
|---|---|---|---|---|---|---|---|
a G – gel; P – precipitate; S – soluble.b Critical gelation concentration (CGC), wt/vol%.c Complex with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. |
|||||||
| Benzene | I | S | I | Cyclohexane | I | I | I |
| Toluene | I | S | I | THF | S | S | S |
| Dichlorobenzene | G (0.13)b | S | G | DMF | S | S | S |
| Bromobenzene | G (0.06) | S | G | DMSO | S | S | S |
| Ethanol | I | I | I | Pyridine | S | S | S |
| Ethyl acetate | I | I | I | Quindine | S | S | S |
| Hexane | I | I | I | Aniline | S | S | S |
| Heptane | I | I | I | N-Methylaniline | S | S | S |
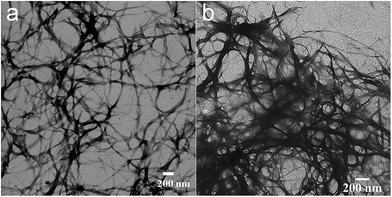 | ||
Fig. 2 TEM images of (a) dichlorobenzene xerogel and (b) the two-component (C8CBTA/C60MZ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) xerogel. 1) xerogel. | ||
As shown above, gelators could self-assemble into thin nanofibers in the gel phase so the driving forces of the formation of 1D aggregates were analyzed using the UV-vis absorption, fluorescence, and FT-IR spectra. Fig. 3a shows the changes in absorption spectra during gelation. The maximal absorption peak in hot solution (120 °C) occurred at 503 nm, which decreased and shifted to 520 nm when the solution was cooled to room temperature and formed gel. An evident red-shift of 17 nm during gelation revealed the J-aggregate formation and the existence of π–π stacking between aromatic moieties.49–52 When the black-red powder of C8CBTA in ODCB was heated to form a clear red solution, a strong red fluorescence with maximum emission at 620 nm was observed (Fig. 3b), which was ascribed to the emission of molecules in monomeric form. When the hot solution cooled, a gradual decrease in emission intensity was observed. Finally, a weak red emission with a maximum at 700 nm was observed in the gel phase. The spectral shifts imply that π–π interaction between aromatic moieties is a major driving force of molecular self-assembly. Because gelator has amide and carboxyl groups, FT-IR spectrum of the xerogel film was measured to study the intermolecular hydrogen bonding. The vibration peak of carboxyl group for C8CBTA gel occurred at 1699 cm−1, and three wide absorption bands ranging from 2760 cm−1 to 2480 cm−1 were observed (Fig. S1†), which demonstrate the dimer structure of the carboxylic acid groups.53 The amide I (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and NH bands appeared at 1650 and 3287 cm−1, respectively. These results imply that all the amide groups were involved in hydrogen bonding.54 The results of absorption and IR spectra proved that π–π interaction and hydrogen bonding play important role in gel formation.
O) and NH bands appeared at 1650 and 3287 cm−1, respectively. These results imply that all the amide groups were involved in hydrogen bonding.54 The results of absorption and IR spectra proved that π–π interaction and hydrogen bonding play important role in gel formation.
Molecular modelling calculation was performed to obtain a stable aggregate to elucidate the self-assembling behavior of C8CBTA in the gel.55 Fig. 4a shows that the two compounds exhibited a J-aggregate in the stable packing structure; this result is consistent with that of the UV-vis absorption spectra. In this π-packing, the intermolecular hydrogen bonds were found between adjacent amide units, which agrees with that of IR spectra. Furthermore, a double hydrogen bonding between carboxyl groups can promote dimer formation (Fig. 4b). These results further proved that the π–π interaction and hydrogen bonding were the driving force for gelation.
Photocurrent generation by the two-component xerogel film
As discussed above, C8CBTA contains two carboxyl groups and exhibits higher LUMO energy level, whereas C60MZ contains an imidazole unit. Given the structures of C8CBTA and C60MZ, they were expected to form hydrogen bonding with the two-component gel with photoinduced electron transfer. When the fraction of C60MZ was less than 100%, the hot dichrolobenzene or bromobenzene solutions of C8CBTA and C60MZ could transform into red gels (Table 2), suggesting the formation of a two-component gel. In other solvents, no gel phase was observed. The maximal absorption peak at 520 nm was independent of the amount of C60MZ (Fig. 5a). This result shows that the addition of C60MZ did not destroy the stacking model (J-aggregate) of C8CBTA, and the charge-transfer complex of C8CBTA and C60MZ did not form into a two-component gel.56–58 Hydrogen bonding between C8CBTA and C60MZ was confirmed by measuring the IR spectra of the two-component xerogels. Fig. S1† shows that the addition of C60MZ did not change the IR peak locations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H, suggesting that the hydrogen bonding between amide groups remained intact. The vibration peak at 1699 cm−1, which is ascribed to the carboxyl dimer, weakened upon the addition of C60MZ, and a peak at 1719 cm−1 corresponding to the free C
O and N–H, suggesting that the hydrogen bonding between amide groups remained intact. The vibration peak at 1699 cm−1, which is ascribed to the carboxyl dimer, weakened upon the addition of C60MZ, and a peak at 1719 cm−1 corresponding to the free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appeared. Moreover, when the equimolar C60MZ was added, the wide absorption bands ranging from 2760 cm−1 to 2480 cm−1 disappeared, indicating that the carboxyl dimer was destroyed and hydrogen bonding between imidazole and carboxyl formed.59 Considering the large diameter (7.1 Å) of C60 and the general π-stacking distance of 3.6 Å, the conversion of stacking model upon the addition of C60MZ is expected.36 Fig. 6 shows that C8CTBA self-assembled into 1D aggregate along with the π-packing direction in the gel phase, and hydrogen bonds formed between carboxyl groups (Fig. 6a). After adding C60MZ, the double hydrogen bonds between carboxyl groups were destroyed and new hydrogen bonds between carboxyl and imidazole formed. Given the strong π–π stacking between aromatic moieties and the hydrogen bonds between amide units, 1D J-aggregate of C8CBTA still existed and half of the carboxyl groups were involved in hydrogen bonding owing to the large diameter of C60MZ. Thus, C60MZ, along with the gelator fibers as electron transport channels, was induced to construct 1D aggregate. In addition, only nanofibers were observed in TEM image (Fig. 2b) of two-component gel (1
O appeared. Moreover, when the equimolar C60MZ was added, the wide absorption bands ranging from 2760 cm−1 to 2480 cm−1 disappeared, indicating that the carboxyl dimer was destroyed and hydrogen bonding between imidazole and carboxyl formed.59 Considering the large diameter (7.1 Å) of C60 and the general π-stacking distance of 3.6 Å, the conversion of stacking model upon the addition of C60MZ is expected.36 Fig. 6 shows that C8CTBA self-assembled into 1D aggregate along with the π-packing direction in the gel phase, and hydrogen bonds formed between carboxyl groups (Fig. 6a). After adding C60MZ, the double hydrogen bonds between carboxyl groups were destroyed and new hydrogen bonds between carboxyl and imidazole formed. Given the strong π–π stacking between aromatic moieties and the hydrogen bonds between amide units, 1D J-aggregate of C8CBTA still existed and half of the carboxyl groups were involved in hydrogen bonding owing to the large diameter of C60MZ. Thus, C60MZ, along with the gelator fibers as electron transport channels, was induced to construct 1D aggregate. In addition, only nanofibers were observed in TEM image (Fig. 2b) of two-component gel (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and C60MZ aggregates did not exist, which also suggests the formation of hydrogen bonding complex. Therefore, photocurrent generation is anticipated if such two-component aggregates were used as active layer.
1) and C60MZ aggregates did not exist, which also suggests the formation of hydrogen bonding complex. Therefore, photocurrent generation is anticipated if such two-component aggregates were used as active layer.
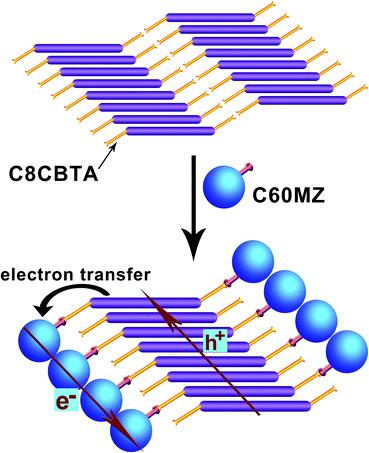 | ||
| Fig. 6 Conversion of stacking model upon the addition of C60MZ and electron transfer from the gelator to C60MZ. Ordered 1D stacking of gelator and C60MZ supply electron and hole transport channels. | ||
The difference between ordered film and disordered film in terms of photocurrent generation was compared by preparing two kinds of complex films. One is a two-component xerogel film (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the other was prepared from their THF solution. The photocurrent measurements for the ITO electrodes coated with active films (which served as the working electrode) were performed using ascorbic acid as a sacrificial electron donor, platinum wire as a counter electrode, and Ag/AgCl as a reference electrode.60 Fig. 5c shows that the photovoltaic cell using xerogel film as active layer exhibited a considerably low current (3.0 × 10−3 μA) in the dark but immediately generated a stable and large amount of photocurrent (0.5 μA) upon irradiation. The current instantly dropped when the illumination was switched off (Fig. 5c). This process is reversible, indicating that the active layer is considerably robust. By contrast, photocurrent was considerably low (0.017 μA) when the disordered film was applied. In addition, we measured the photocurrents of the electrode with xerogel film and blank electrode, and found that current is less than 2.0 × 10−8 A. This result suggests that the existence of excellent p- and n-channels is important in generating larger photocurrent. Moreover, a complex xerogel film with a larger thickness was prepared and its photocurrent was measured. As shown in Fig. 5c, stable current could be observed under light irradiation, but it is weaker than that of thin one, indicating thick film is not a better choice to act as active layer because organic molecules always possess small hole mobility and short exciton diffusion length.
1) and the other was prepared from their THF solution. The photocurrent measurements for the ITO electrodes coated with active films (which served as the working electrode) were performed using ascorbic acid as a sacrificial electron donor, platinum wire as a counter electrode, and Ag/AgCl as a reference electrode.60 Fig. 5c shows that the photovoltaic cell using xerogel film as active layer exhibited a considerably low current (3.0 × 10−3 μA) in the dark but immediately generated a stable and large amount of photocurrent (0.5 μA) upon irradiation. The current instantly dropped when the illumination was switched off (Fig. 5c). This process is reversible, indicating that the active layer is considerably robust. By contrast, photocurrent was considerably low (0.017 μA) when the disordered film was applied. In addition, we measured the photocurrents of the electrode with xerogel film and blank electrode, and found that current is less than 2.0 × 10−8 A. This result suggests that the existence of excellent p- and n-channels is important in generating larger photocurrent. Moreover, a complex xerogel film with a larger thickness was prepared and its photocurrent was measured. As shown in Fig. 5c, stable current could be observed under light irradiation, but it is weaker than that of thin one, indicating thick film is not a better choice to act as active layer because organic molecules always possess small hole mobility and short exciton diffusion length.
Conclusions
A new gelator with strong electron-withdrawing benzothiadiazole, electron-donating N-octyl-3-amidocarbazole and two carboxyl groups as hydrogen bond donor was designed and synthesized. When induced by intermolecular hydrogen bonds and π–π interaction, this gelator could self-assemble into 1D J-aggregate in its gel phase. The maximum absorption peak of the gel located at 520 nm. Moreover, a fullerene with one imidazole unit as an electron acceptor was synthesized and was able to form two-component hydrogen-bonding gels with gelator resulting from the hydrogen bonds between carboxyl and imidazole moieties. In the two-component gel, the 1D aggregate of fullerene derivative, which serves as an electron transport channel, formed along the gel fibers. Upon irradiation, larger amount of photocurrent was generated by the xerogel film compared with the disordered film. This result reveals that the strong electron-withdrawing group possibly promoted longer absorption wavelength, and the 1D arrangement of electron acceptor and donor is important for the large photocurrent generation. Future works will focus on the synthesis of gelator with longer absorption wavelength.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21103067, and 21374041), the Youth Science Foundation of Jilin Province (20130522134JH), the Open Project of the State Key Laboratory of Supramolecular Structure and Materials (SKLSSM2015014), the Open Project of State Laboratory of Theoretical and Computational Chemistry (K2013-02).Notes and references
- L. Basabe-Desmonts and D. N. Reinhoudt, Chem. Soc. Rev., 2007, 36, 993–1017 RSC.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS PubMed.
- Y. Ren, A. M. Hiszpanski, L. Whittaker-Brooks and Y. Loo, ACS Appl. Mater. Interfaces, 2014, 6, 14533–14542 CAS.
- S. Bureekaew, S. Horike, M. Higuchi, M. Mizuno, T. Kawamura, D. Tanaka, N. Yanai and S. Kitagawa, Nat. Mater., 2009, 8, 831–836 CrossRef CAS PubMed.
- T. E. Kaiser, H. Wang, V. Stepanenko and F. Würthner, Angew. Chem., Int. Ed., 2007, 46, 5541–5544 CrossRef CAS PubMed.
- X. Ma, Y. Zhang, Y. Zheng, Y. Zhang, X. Tao, Y. Che and J. Zhao, Chem. Commun., 2015, 51, 4231–4233 RSC.
- C. Park, J. E. Park and H. C. Choi, Acc. Chem. Res., 2014, 47, 2353–2364 CrossRef CAS PubMed.
- X. Wang, C. Drew, S.-H. Lee, K. J. Senecal, J. Kumar and L. A. Samuelson, Nano Lett., 2002, 2, 1273–1275 CrossRef CAS.
- X. Chen, C. K. Y. Wong, C. A. Yuan and G. Zhang, Sens. Actuators, B, 2013, 177, 178–195 CrossRef CAS PubMed.
- Y. S. Zhao, J. Wu and J. Huang, J. Am. Chem. Soc., 2009, 131, 3158–3159 CrossRef CAS PubMed.
- K. S. Park, B. Cho, J. Baek, J. K. Hwang, H. Lee and M. M. Sung, Adv. Funct. Mater., 2013, 23, 4776–4784 CAS.
- N. M. Tucker, A. L. Briseno, O. Acton, H. Yip, H. Ma, S. A. Jenekhe, Y. Xia and A. K.-Y. Jen, ACS Appl. Mater. Interfaces, 2013, 5, 2320–2324 CAS.
- C. Luo, R. Huang, R. Kevorkyants, M. Pavanello, H. He and C. Wang, Nano Lett., 2014, 14, 1596–1602 CrossRef CAS PubMed.
- M. Hirade, H. Nakanotani, M. Yahiro and C. Adachi, ACS Appl. Mater. Interfaces, 2011, 3, 80–83 CAS.
- Q. Liao, Z. Xu, X. Zhong, W. Dang, Q. Shi, C. Zhang, Y. Weng, Z. Li and H. Fu, J. Mater. Chem. C, 2014, 2, 2773–2778 RSC.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed.
- N. Yan, Z. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, J. Am. Chem. Soc., 2013, 135, 8989–8999 CrossRef CAS PubMed.
- S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766–1776 CrossRef CAS PubMed.
- Z. Zhao, J. W. Y. Lam and B. Z. Tang, Soft Matter, 2013, 9, 4564–4579 RSC.
- S. K. Samanta and S. Bhattacharya, Chem. Commun., 2013, 49, 1425–1427 RSC.
- S. S. Babu, K. K. Kartha and A. Ajayaghosh, J. Phys. Chem. Lett., 2010, 1, 3413–3424 CrossRef CAS.
- X. Yu, L. Chen, M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346–5371 RSC.
- H. Najafov, B. Lee, Q. Zhou, L. C. Feldman and V. Podzorov, Nat. Mater., 2010, 9, 938–943 CrossRef CAS PubMed.
- P. Xue, Q. Xu, P. Gong, C. Qian, A. Ren, Y. Zhang and R. Lu, Chem. Commun., 2013, 49, 5838–5840 RSC.
- H. Peng, L. Ding, T. Liu, X. Chen, L. Li, S. Yin and Y. Fang, Chem.–Asian J., 2012, 7, 1576–1582 CrossRef CAS PubMed.
- X. Zhang, X. Liu, R. Lu, H. Zhang and P. Gong, J. Mater. Chem., 2012, 22, 1167–1172 RSC.
- K. K. Kartha, S. S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834–4841 CrossRef CAS PubMed.
- G. Hong, C. Qian, P. Xue, X. Liu, Q. Wang, M. Liu, P. Gong and R. Lu, Eur. J. Org. Chem., 2014, 6155–6162 CrossRef CAS PubMed.
- J. P. Hong, M. C. Um, S. R. Nam, J. I. Hong and S. Lee, Chem. Commun., 2009, 310–312 RSC.
- R. Marty, R. Nigon, D. Leite and H. Frauenrath, J. Am. Chem. Soc., 2014, 136, 3919–3927 CrossRef CAS PubMed.
- K. Sugiyasu, S. Kawano, N. Fujita and S. Shinkai, Chem. Mater., 2008, 20, 2863–2865 CrossRef CAS.
- A. Wicklein, S. Ghosh, M. Sommer, F. Würthner and M. Thelakkat, ACS Nano, 2009, 3, 1107–1114 CrossRef CAS PubMed.
- T. V. Pho, F. M. Toma, M. L. Chabinyc and F. Wudl, Angew. Chem., Int. Ed., 2013, 52, 1446–1451 CrossRef CAS PubMed.
- X. Yang, G. Zhang, D. Zhang and D. Zhu, Langmuir, 2010, 26, 11720–11725 CrossRef CAS PubMed.
- I. D. Tevis, W. Tsai, L. C. Palmer, T. Aytun and S. I. Stupp, ACS Nano, 2012, 6, 2032–2040 CrossRef CAS PubMed.
- P. Xue, R. Lu, L. Zhao, D. Xu, X. Zhang, K. Li, Z. Song, X. Yang, M. Takafuji and H. Ihara, Langmuir, 2010, 26, 6669–6675 CrossRef CAS PubMed.
- P. Xue, Q. Xu, P. Gong, C. Qian, Z. Zhang, J. Jia, X. Zhao, R. Lu, A. Ren and T. Zhang, RSC Adv., 2013, 3, 26403–26411 RSC.
- P. Xue, P. Wang, B. Yao, J. Sun, P. Gong, Z. Zhang, C. Qian and R. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 21426–21434 CAS.
- J. H. Jia, K. Y. Cao, P. C. Xue, Y. Zhan, H. P. Zhou and R. Lu, Tetrahedron, 2012, 68, 3626–3632 CrossRef CAS PubMed.
- J. S. Stover, J. Shi, W. Jin, P. K. Vogt and D. L. Boger, J. Am. Chem. Soc., 2009, 131, 3342–3348 CrossRef CAS PubMed.
- H. Jintoku, M. Yamaguchi, M. Takafuji and H. Ihara, Adv. Funct. Mater., 2014, 24, 4105–4112 CrossRef CAS PubMed.
- H. Jintoku, M. Takafuji, R. Oda and H. Ihara, Chem. Commun., 2012, 48, 4881–4883 RSC.
- C. Zhao, A. Wakamiya, Y. Inukai and S. Yamaguchi, J. Am. Chem. Soc., 2006, 128, 15934–15935 CrossRef CAS PubMed.
- Y. Xu, P. C. Xue, D. F. Xu, X. F. Zhang, X. L. Liu, H. P. Zhou, J. H. Jia, X. C. Yang, F. Y. Wang and R. Lu, Org. Biomol. Chem., 2010, 8, 4289–4296 CAS.
- P. Xue, J. Sun, B. Yao, P. Gong, Z. Zhang, C. Qian, Y. Zhang and R. Lu, Chem.–Eur. J., 2015, 21, 4712–4720 CrossRef CAS PubMed.
- L. Lu and L. Yu, Adv. Mater., 2014, 26, 4413–4430 CrossRef CAS PubMed.
- X. Yan, Y. Cui, Q. He, K. Wang and J. Li, Chem. Mater., 2008, 20, 1522–1526 CrossRef CAS.
- E. L. Bonifazi, V. C. Edelsztein, G. O. Menéndez, C. S. López, C. C. Spagnuolo and P. H. D. Chenna, ACS Appl. Mater. Interfaces, 2014, 6, 8933–8936 CAS.
- F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef PubMed.
- H. Wu, L. Xue, Y. Shi, Y. Chen and X. Li, Langmuir, 2011, 27, 3074–3082 CrossRef CAS PubMed.
- K. Narasimha and M. Jayakannan, ACS Appl. Mater. Interfaces, 2014, 6, 19385–19396 CAS.
- V. Bhalla, A. Gupta, M. Kumar, D. S. S. Rao and S. K. Prasad, ACS Appl. Mater. Interfaces, 2013, 5, 672–679 CAS.
- S. Wang, P. Xue, P. Wang and B. Yao, New J. Chem., 2015, 39, 6874–6881 RSC.
- N. Dey, S. K. Samanta and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 8394–8400 CAS.
- G. Srinivas and M. L. Klein, Nanotechnology, 2007, 18, 205703 CrossRef.
- R. K. Das, S. Banerjee, G. Raffy, A. D. Guerzo, J. Desvergne and U. Maitra, J. Mater. Chem., 2010, 20, 7227–7235 RSC.
- Y. Liu, N. Zheng, H. Li and B. Yin, Soft Matter, 2013, 9, 5261–5269 RSC.
- P. Bairi, B. Roy, P. Routh, K. Sen and A. K. Nandi, Soft Matter, 2012, 8, 7436–7445 RSC.
- C. Bao, R. Lu, M. Jin, P. Xue, C. Tan, G. Liu and Y. Zhao, Org. Biomol. Chem., 2005, 3, 2508–2512 CAS.
- Y. Cho, T. K. Ahn, H. Song, K. S. Kim, C. Y. Lee, W. S. Seo, K. Lee, S. K. Kim, D. Kim and J. T. Park, J. Am. Chem. Soc., 2005, 127, 2380–2381 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Absorption and fluorescence data in different solvents, and FT-IR spectra of gels. See DOI: 10.1039/c5ra15236d |
| This journal is © The Royal Society of Chemistry 2015 |





