Mechanistic insight to acidity effects of Ga/HZSM-5 on its activity for propane aromatization†
He Xiaoab,
Junfeng Zhanga,
Peng Wangab,
Zhenzhou Zhangab,
Qingde Zhanga,
Hongjuan Xiea,
Guohui Yanga,
Yizhuo Hana and
Yisheng Tan*a
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China. E-mail: tan@sxicc.ac.cn; Fax: +86-351-4044287; Tel: +86-351-4044287
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 14th October 2015
Abstract
Ga-modified HZSM-5 precursors, containing 1 wt% Ga, were first prepared using the incipient wetness impregnation method, and then subjected to one or three-times consecutive treatment by cycles of reduction in hydrogen and re-oxidation in air. The resulting Ga/HZSM-5 catalysts were characterized by N2 physical adsorption, ICP-AES, DRIFT, Py-FTIR, NH3-TPD, H2-TPR, XPS, DRIFT-TPSR and MS-TPSR techniques to obtain clear mechanistic details that the acidity of these Ga/HZSM-5 catalysts affected their activity for propane aromatization. The characterization data suggested that the impregnated introduction of Ga to ZSM-5 zeolite and subsequent reduction–oxidation treatment led to a great decrease in the number of Brønsted acid sites (BAS) and promoted the formation of strong Lewis acid sites (LAS) attributed to highly dispersed Ga species. The formed strong LAS specifically promoted the dehydrogenation steps during propane aromatization, whereas the original BAS were responsible for the whole aromatization process. The TPSR results suggested that propane was converted to propylene through the dehydrogenation on BAS and strong LAS, and simultaneously converted to ethylene through the β-scission on BAS at a low temperature. With the elevation of temperature, the generated propylene and ethylene on the strong BAS and the strong LAS were further converted into BTX aromatics accompanied by hydrogen release. It was plausible that the highly efficient synergy between BAS and strong LAS could result in lower aromatization temperatures and more product of benzene (β state) over the Ga/HZSM-5 catalysts than that over the Ga-modified HZSM-5 precursors.
1. Introduction
The worldwide supply of liquefied petroleum gas (LPG) was about 140 million metric tonnes in 1990 and rose to about 275 million metric tonnes by year 2014.1 Moreover, urgent demand of BTX (benzene, toluene and xylene) has drawn the attention of academic and industrial sectors to deeply investigate the conversion of lower alkanes into these aromatics. Therefore, propane (light alkane) aromatization has been extensively studied in recent years. Previous studies2–17 focused on the bifunctional Ga modified ZSM-5 catalysts for aromatization because of their superior catalytic performance compared to Zn and Pt modified ZSM-5 catalysts.4,8 It was found that incorporation of Ga to ZSM-5, which was prepared using different methods, such as hydrothermal in situ synthesis and post synthesis treatment (ion-exchange, impregnation, chemical vapour deposition [CVD] and reduction–oxidation treatment), could adjust the acidity of the Ga/ZSM-5 catalysts. Moreover, the varied acidity, in turn, had a significant effect on the propane aromatization performance of the catalysts.4,6 Choudhary et al.18 found that the Si/Ga and Si/Al ratios of H-gallosilicate (MFI) prepared using the in situ hydrothermal synthesis method strongly affected its acidity/acid strength distribution, therefore inducing a dramatic difference of catalytic activity/selectivity in propane aromatization. The highest propane conversion and selectivity of aromatics over these HMFI catalysts came to about 75% and 80%, respectively. Moreover, Al-Yassir et al.16 further reported that, through de-galliation from the ZSM-5 framework in hydrothermal synthesis process, Lewis acid sites (LAS) associated with highly dispersed and reducible extra-framework Ga2O3 were generated on the catalyst surface, which finally resulted in high activity of propane aromatization. The optimal propane conversion and selectivity of BTX achieved was 42.0% and 52.0%, respectively. El-Malki et al.19 prepared a highly efficient Ga/ZSM-5 catalyst for aromatization using a CVD method together with subsequent water treatment. They found that the subsequent treatment of the catalyst with water could create secondary strong Brønsted acid sites (BAS), contributing to the aromatization activity of Ga/ZSM-5 catalyst. Abdul Hamid et al.20 revealed that the reductive atmosphere could promote the migration of Ga species and formation of active Ga species, which further affected the acidity of Ga/HZSM-5 catalysts. These active Ga species and BAS over ZSM-5 had a synergistic effect on the catalytic performance of the Ga/HZSM-5 catalysts. The highest propane conversion and selectivity of BTX were not more than 10% and 40%, respectively. Nowak et al.3 investigated the effect of H2–O2 cycles on the activity of Ga/HZSM-5 prepared using the ion-exchange method. They found the synergistic effect between the reduced state Ga species generated by H2–O2 treatment and BAS over the ZSM-5 zeolites contributed to the highest selectivity of aromatics, which was 19.1% at a high conversion (97–99%) for the aromatization of propane at 823 K using Ga/(0.5) as catalysts. Through the structure–activity correlations between the acidity of Ga/ZSM-5 catalyst and its activity, Rodrigues et al.3,10,11,20 thought that the reduction–oxidation treatment promoted the formation of highly dispersed oxidic gallium species, which were anchored to ion-exchange sites of the zeolite framework, and contributed to the strong LAS. Moreover, it was also found that the synergy between LAS and BAS led to alkane aromatization activity rather than exclusive effect of LAS or BAS in the reaction. It was believed that the reducibility of strong Lewis acid species facilitated the formation of reduced gallium species, which was the actual active site in alkane activation. As observed, initial rates of propane conversion were at least 20 times higher for the Ga-containing catalysts obtained by them than the respective zeolite, but were almost independent of gallium content. Considering the aromatization activities alone, gallium impregnation led to catalysts that were approximately 200 times more active in the case of the TZ35 series because of synergy between LAS and BAS.In our previous study,13 a highly efficient Ga/ZSM-5 catalyst was prepared by formic acid impregnation and in situ treatment for propane aromatization. This novel treatment process promoted the formation of strong LAS attributed to highly dispersed (GaO)+ species through decomposition of intermediate species and the interaction between Ga2O3 and water steam. Based on the investigation of the catalytic system, it was thought that the high catalytic activity of this catalyst was attributed to the synergistic effect between strong LAS and BAS. The highest propane conversion and selectivity of BTX on the Ga/HZSM-5 catalyst we prepared by this novel method were 53.6% and 58.0%, respectively. It is obvious that the activities of the catalysts prepared using different methods are different. Considering the distinction between test conditions, we are reluctant to make a certain conclusions on which preparation method is preferential to obtain good catalytic performance. However, it is widely acknowledged that the presence of highly dispersed Ga on HZSM-5, which is obtained through special treatment process such as reduction–oxidation operation, is favorable for propane aromatization. This is evidenced from our present study. In the present study, the preparation process of the Ga/HZSM-5 and the formation process of active Ga species are different from the previous study.13 Ga-modified HZSM-5 precursors were first prepared using an incipient wetness impregnation method, and then subjected to one or three-time consecutive treatment by cycles of reduction in hydrogen and re-oxidation in air. The reduction–oxidation treatment could promote the migration of these Ga species into the zeolite and the formation of highly dispersed (GaO)+ species is inferred from our techniques and previous research.3,11 It was apparent that these two different preparation processes (previous treatment and present treatment) promoted the formation of (GaO)+ species. Wherein, propane conversion and selectivity of the Ga/HZSM-5 catalyst prepared using reduction–oxidation method showed a significant increase of 15.2–22.3% and 9.5–10.9%, respectively, in contrast to that of the Ga/HZSM-5 catalyst prepared using traditional impregnation methods at the same test conditions. The superior activity of the Ga/HZSM-5 catalyst prepared using the reduction–oxidation method was attributed to the synergistic effect between the strong LAS generated by the (GaO)+ species and the BAS. Conclusively, it is apparent that the reduction–oxidation treatment improves the activity of Ga containing HZSM-5 zeolites greatly. Simultaneously, it is obvious that, if one looks forward to a high activity of Ga/ZSM-5 catalyst for propane aromatization, obtaining a suitable amount of BAS and LAS related to Ga species through adopting some various/special preparation and treatment methods is necessary.
The previous study focused on a novel proposed preparation method for catalyst and the probable formation route of highly active GaO+ species,13 whereas in the present study, we concentrated on the systematic investigation of how the acidity of Ga/HZSM-5 catalyst affected the evolution of reactants and intermediates (such as β-scission of propane to methane and ethylene and dehydrogenation of propane to propylene) on specific acid sites (BAS and LAS) of Ga/HZSM-5 as well as the sorption/desorption process of reactants and intermediates on these acid sites. In other words, we paid more attention to the relationship between the acid sites (LAS and BAS) and specific reactions as well as the sorption/desorption of reactants and intermediates, as the systematic investigation on this research was not reported to us. Therefore, in this study, we in-detail rationalized the results in terms of correlations between the acidity (Lewis acidity and Brønsted acidity) of Ga/HZSM-5 catalyst and its reactivity (specific reactions as well as the sorption/desorption process of reactants and intermediates) in propane aromatization by various characterizations of the catalysts such as N2 physical adsorption, ICP-AES, DRIFT, Py-FTIR, NH3-TPD, H2-TPR, XPS, DRIFT-TPSR and MS-TPSR techniques. Furthermore, the mechanistic details that the acidity of the Ga/HZSM-5 catalysts affected their activity for propane aromatization were deeply illustrated.
2. Experimental section
2.1 Catalyst preparation
HZSM-5 zeolites (silica to aluminum mole ratio = 19) were bought from Nankai University. Ga was incorporated into this zeolite by incipient wetness impregnation with gallium nitrate solution (1 wt% Ga loading). The impregnated sample was calcined at 550 °C for 5 h under static air. Thus, the Ga/IM catalyst was gained accordingly. Subsequently, 0.15 g of Ga/IM catalyst was placed in a micro fixed-bed reactor and then subjected to the reduction–oxidation treatment. Herein, reduction was performed under a hydrogen flow of 15 mL min−1 at 540 °C for 1 h and re-oxidation was carried out under an air flow of 15 mL min−1 for 1 h. The resulting catalysts were denoted as Ga/IMRO1C and Ga/IMRO3C based on one and three times reduction–oxidation treatment to Ga/IM, respectively.2.2 Catalyst characterization
The textural properties of catalyst were measured by N2 physical sorption at 77 K using a Tristar 3000 machine. Surface areas were calculated by the BET method and micro-, meso-, and macro-pore volumes were calculated by the t-plot method.The powder X-ray diffraction (XRD) patterns were obtained on a Rigaku MiniFlex II X-ray diffractometer using CuKα radiation. The anode was operated at 40 kV and 40 mA. The 2θ angles were scanned from 5° to 60°.
TEM micrographs were obtained by a JEOL-JEM-2100F transmission electron microscope operating at 200 kV.
Diffuse reflectance infrared Fourier transform spectra (DRIFT) measurements were obtained using a Bruker Tensor 27 instrument with a MCT detector (64 scans, 4 cm−1). 20 mg of catalyst was weighed and placed in an infrared cell with KBr windows for in situ treatments. The DRIFT spectra were obtained after treating the catalyst at 300 °C for 1 h with a flow of argon.
Pyridine-adsorbed Fourier transform infrared (Py-FTIR) spectroscopy was used to determine the amount of BAS and LAS using Bruker Tensor 27 equipment. 20 mg of catalyst was pressed into a regular wafer (R = 1.3 cm−1) and placed in an infrared cell. The infrared spectrum was obtained after sample treatment at 400 °C for 2 h under vacuum, and this spectrum was used as background for the adsorbed pyridine experiments. Pyridine was then adsorbed to a 5.0 × 10−2 Pa equilibrium pressure at 40 °C. FTIR spectra were obtained after consecutive evacuation at 150, 250, 350 and 450 °C.
The temperature-programmed desorption of ammonia (NH3-TPD) was used to test the amount and strength of the acid sites of the as-prepared catalysts in TP-5080 chemisorption instrument. The catalyst (100 mg) was pre-treated at 500 °C under a flow of N2 (30 mL min−1) for 2 h and then cooled to 100 °C. Then, NH3 was introduced into the flow system. The TPD spectra were obtained at a ramp rate of 10 °C min−1 from 100 °C to 700 °C.
The H2-temperature-programmed reduction (H2-TPR) was carried out to study reducibility of the catalysts with chemisorption instrument (TP-5080). The catalyst (100 mg) was pre-treated at 500 °C under a flow of N2 (32 mL min−1) for 1 h and then cooled to 100 °C, then the flow was changed to a H2/N2 = 0.09 mixture (35 mL min−1). The temperature-programmed reduction was performed between 100 °C and 900 °C with a ramping rate of 10 °C min−1.
X-ray photoelectron spectroscopy (XPS) was used to analyze the change of surface composition measured by AXIS ULTRA DLD equipment. The following photoelectron lines were obtained: Ga2p3/2, Ga2p1/2, Ga3d, Si2p, Al2p, O1s, O2s and C1s. The binding energy values were corrected for charging effect by referring to the adventitious C1s line at 284.5 eV.
Diffuse reflectance infrared spectra (DRIFTS) investigation of temperature-programmed surface reaction (TPSR) was carried out in an in situ reaction cell. The catalyst (20 mg) was pre-treated at 400 °C under a flow of argon (30 mL min−1) for 1 h and then cooled to 50 °C. Furthermore, propane flow was introduced to the reaction cell for absorption for 0.5 h and then switched to the argon flow (30 mL min−1) to remove the physical adsorption of propane (blowing for 0.5 h). The above-treated sample was heated from 50 °C to 450 °C at a ramping rate of 3 °C min−1; moreover, its diffuse reflectance infrared spectra were obtained.
Mass spectrum investigation of temperature programmed surface reaction (MS-TPSR) was carried out on a chemisorption instrument (TP-5080) and OMNI star. The catalyst (100 mg) was first pre-treated at 500 °C for 1 h under a flow of argon (30 mL min−1) and then cooled to 50 °C. After that, propane was introduced into the flow system until saturation sorption (about 0.5 h). The sample was heated again from 50 °C to 650 °C with a ramping rate of 3 °C min−1 and the effluents from the reactor were analysed by an on-line mass spectrometer synchronously.
2.3 Catalyst evaluation
The catalyst test, as our previous illustration,13 was carried out in a horizontal quartz tube fixed-bed reactor at T = 540 °C, P = 100 kPa, WHSV = 6000 mL (g−1 h−1) and with a N2/C3H8 molar ratio of 2. Outlet gas was analysed after 0.5–4.5 h reaction.The products were analysed by an online gas chromatograph (HUAAI GC 9560) equipped with a flame ionization detector (FID) with a HP-INNOWAX capillary column, an off-line gas chromatograph (HUAAI GC 9560) equipped with a FID with an Al2O3 packed column, and an offline gas chromatograph (East & West GC 4000A) equipped with a thermal conductivity detector (TCD) with a carbon molecular sieves packed column, respectively. The propane conversion and product selectivity were calculated using the following eqn (1) and (2).
 | (1) |
 | (2) |
3. Results and discussion
3.1 Catalyst composition and textural properties
The pore volume and BET surface areas (SBET) of different catalysts are obtained based on the sorption isotherms of N2 condensation at 77 K and the results are listed in Table 1. As observed, the micropore surface area and micropore volume of the Ga-containing ZSM-5 zeolites decreases compared to that of HZSM-5, especially for Ga/IMRO1C and Ga/IMRO3C treated by reduction–oxidation process, the decrease is more severe. It is thought that the decrease of micropore surface area and micropore volume is probably due to the pore blockage resulting from the deposition of extra-framework gallium and aluminum species within the micropores.11,13 In addition, compared with surface areas and porous volumes of meso and macropores of Ga/IM, those of Ga/IMRO1C and Ga/IMRO3C show a slight increase. This indicates that the reduction–oxidation treatment somewhat has a potential effect on the expansion of the micropores. TEM characterization of these catalysts is also used to visualize the pore structure. As shown in Fig. S1a–d,† Ga/IMRO1C and Ga/IMRO3C show regular hexagonal prisms such as Ga/IM and HZSM-5. It suggests that impregnation and reduction–oxidation treatment slightly affect the morphologies of ZSM-5 zeolites. At the same time, it is found that no irregular pore structures as well as messy species are created after impregnation and reduction–oxidation treatment, as observed in Fig. S1a–d.† In combination of BET data, it is thought that the pore structures of the zeolites are slightly affected and most of them could be preserved during catalyst preparation and pre-treatment.| Sample | SBETa (m2 g−1) | Smicroa (m2 g−1) | Sext (m2 g−1) | Vtotalb (m2 g−1) | Vmicrob (m2 g−1) | Vext (m2 g−1) |
|---|---|---|---|---|---|---|
| a Determined by t-method.b Determined by v adsorbed at p/p0 = 0.97. | ||||||
| HZSM-5 | 420 | 324 | 95.8 | 0.185 | 0.129 | 0.056 |
| Ga/IM | 400 | 301 | 99.1 | 0.180 | 0.121 | 0.059 |
| Ga/IMRO1C | 384 | 275 | 109 | 0.177 | 0.111 | 0.066 |
| Ga/IMRO3C | 381 | 270 | 111 | 0.177 | 0.109 | 0.068 |
The XRD patterns of the as-prepared samples reveal the typical characteristics of HZSM-5 (Fig. S2†). However, no XRD reflections ascribed to segregated bulk Ga2O3 or framework gallium species can be found on these catalyst.21 It is probably due to low Ga content, highly dispersed ions and/or extra-crystalline oxide-type domain with sizes below 4.0 nm on the external surface.16 The crystallinities of these materials are determined on the intensity of characteristic peaks (6.9–9.1° and 22.6–24.8°) in XRD patterns. The peak intensity of parent HZSM-5 is highest and its crystallinity is defined as 100%. The crystallinities of other materials depend on the ratios of their strength value of characteristic peaks divided by that of parent HZSM-5. Based on that, crystallinities of the Ga/IM (55.8%), Ga/IMRO1C (66.4%) and Ga/IMRO3C (72.4%) can be obtained. It is found that crystallinity of the Ga/IM dramatically decreases to about 55.8% from original 100% of ZSM-5. However, the crystal can be recovered partly through the reduction–oxidation treatment as observed from 66.4% crystallinity of Ga/IMRO1C and 72.4% crystallinity of Ga/IMRO3C, respectively. It is believed that the reduction–oxidation treatment is beneficial for preserving the pore structures of the zeolites.
Table 2 shows the bulk and surface compositions of as-prepared catalysts based on measurements of ICP-AES and XPS. As observed, the surface Si/Al ratios of these catalysts decrease and are lower than that of the bulk with the introduction of Ga, indicating the enrichment of aluminum on catalyst surface. The increase of aluminum implies that the framework of the Ga modified ZSM-5 zeolites suffers from de-alumination caused by introduction of Ga and the subsequent reduction–oxidation treatment. Therefore, the de-alumination of Ga/IMRO1C and Ga/IMRO3C is more severe. Furthermore, it can be observed that the ratios of Si/Ga of the catalysts' surface are lower than that of its bulk. This indicates that the Ga species are enriched on the surface of these catalysts, whereas higher surface Si/Ga ratios of Ga/IMRO1C and Ga/IMRO3C than that of Ga/IM indicates that the reduction–oxidation treatment is in favor of promoting the entry of the surface Ga into the intra-crystalline region/channels of the zeolite.
3.2 DRIFT
To observe the OH stretching change of special groups that exist on the catalyst surface, the as-prepared catalysts are investigated by means of DRIFT, and the results are shown in Fig. 1. In the region from 3400 cm−1 to 4000 cm−1, four bands are observed on four samples.3,6 Wherein, (i) the band at 3601 cm−1 is ascribed to the stretching vibration of Si(OH)Al bridged bonds, which is associated with the zeolite BAS;6,11 (ii) the band at about 3650 cm−1 is ascribed to the extra-framework aluminium OH group, which is associated with zeolite LAS;2 (iii) the band at 3671 cm−1 is assigned to the extra-framework gallium OH group, which is associated with weak LAS;2,22 and (iv) the band at 3737 cm−1 is attributed to the terminal Si–OH group. As observed in Fig. 1, the intensity of the peak at 3601 cm−1 on Ga/IM decreases with Ga incorporation to HZSM-5, suggesting that the number of BAS decreases. It is plausible that the decrease results from exchange of the partially acidic protons with ionic gallium species or de-alumination of ZSM-5 zeolites.7 Moreover, a further reduction in intensity of the 3601 cm−1 peak occurs on Ga/IMRO1C and Ga/IMRO3C, implying that the reduction–oxidation treatment also leads to the decrease of BAS, whereas the intensity of the peak close to 3650 cm−1 on Ga-containing ZSM-5 zeolites, especially for Ga/IMRO1C and Ga/IMRO3C, increases in contrast with that on HZSM-5; however, the spectra indicate that de-alumination has occurred with Ga incorporation to HZSM-5 and is aggravated by subsequent reduction–oxidation treatment, as previously verified by the results of XPS in Table 2. Furthermore, an increase in intensity of the peak at 3671 cm−1 on Ga-containing ZSM-5 zeolites is not obvious, probably because part of the peak is concealed by the peak of Al–OH and more surface Ga enters into the intra-crystalline region/channels of zeolites by reduction–oxidation treatment. | ||
| Fig. 1 DRIFT spectra in the OH stretching region of the as-prepared catalysts. (a) HZSM-5; (b) Ga/IM; (c) Ga/IMRO1C; and (d) Ga/IMRO3C. | ||
3.3 Py-FTIR
FTIR spectra of pyridine adsorption on different catalysts are obtained to identify the BAS and LAS, and the quantified results of different types of acid sites are shown in Fig. 2. It is noted that there are three adsorption peaks of pyridine at ∼1450 cm−1, ∼1490 cm−1 and ∼1540 cm−1, which are assigned to the characteristic bands of LAS, the co-contribution of LAS and BAS, and BAS, respectively (as shown in Fig. 2A).11 As observed in Fig. 2B, the number of BAS decreases with the elevated evacuation treatment temperature for all as-prepared samples. It implies that both weak and strong BAS are present on these catalysts. Compared with HZSM-5, Ga/IM shows a slight decrease in the number of BAS. Furthermore, the decrease in BAS is greatly deteriorated when Ga/IM is treated with reduction–oxidation as that of Ga/IMRO1C and Ga/IMRO3C in Fig. 2B. The results are consistent with the previous infrared results in the OH stretching region. The concentration of pyridine adsorption on LAS of the catalysts as a function of evacuation temperature is shown in Fig. 2C. The figure shows that pyridine adsorbed on LAS of HZSM-5 disappears substantially after evacuation at 250 °C. However, as for Ga/IMRO1C and Ga/IMRO3C, pyridine absorption is still present even at temperatures as high as 450 °C, indicating that strong LAS are generated as a result of Ga incorporation via the subsequent reduction–oxidation treatment. Previous study11,23–26 reported that the incorporation of highly dispersed Ga species to ZSM-5 zeolites could promote the formation of strong LAS, which improved dehydrogenation ability of the catalysts. That is to say, the reduction–oxidation treatment to Ga/IM enhances the formation of highly dispersed Ga species contributing to strong LAS. Moreover, the numbers of BAS and the numbers of LAS over the Ga/HZSM-5 catalysts prepared by reduction–oxidation treatment are higher than the Ga/HZSM-5 catalysts prepared by previous treatment (formic acid impregnation and in situ treatment).13 That is because the reduction–oxidation treatment leads to less framework damage to the catalysts. From Fig. 2D, it can be observed that pure HZSM-5 has the largest ratio of Brønsted acid concentration/Lewis acid concentration, indicating that large amounts of BAS are present on its surface. However, the ratio decreases with Ga incorporation (such as Ga/IM). Furthermore, the decrease is very dramatic after the subsequent reduction–oxidation treatment as Ga/IMRO1C and Ga/IMRO3C show. Previous research4,27 suggested that BAS of HZSM-5 are exclusive active sites for propane aromatization during the whole reaction, whereas the LAS associated with extra-framework aluminium OH do not contribute to the propane aromatization. However, our prepared Ga/IMRO1C and Ga/IMRO3C exhibited higher conversion of propane and selectivity of BTX than Ga/IM and HZSM-5, as shown in Fig. 3. In combination with other4,11 and our previous reports,13 it can be deduced that the formed strong LAS by Ga modification to HZSM-5 is equally important for propane aromatization. It can be concluded, apparently, that the synergy effect between strong LAS is related to highly dispersed Ga species and BAS on Ga/IMRO1C and Ga/IMRO3C contributes to propane aromatization activity rather than the exclusive effect of BAS.3.4 NH3-TPD
Shown in Fig. 4 are the NH3-TPD profiles obtained over the as-prepared catalysts. As observed, all catalysts show two desorption peaks of NH3 (centred at 150–340 °C and 360–570 °C), which are associated with weak acid sites and strong acid sites, respectively. Compared with two desorption peaks on HZSM-5, the Ga-containing ZSM-5 zeolites don't show an obvious shift indicating that the incorporation of Ga to HZSM-5 induces little change of acid strength. On the other hand, one can note that the intensity of desorption peaks at 150–340 °C on the Ga-containing samples show a slight decrease in comparison with that on HZSM-5, whereas the intensity of the desorption peak at 360–590 °C on Ga/IMRO1C and Ga/IMRO3C dramatically decreases. It also can be further confirmed based on the data of the quantified acid sites as listed in Table S1.† The abovementioned result suggests that the present method of preparing catalyst can obviously reduce the amount of total acid sites, and most of the decrease is caused by the removal of partial strong acid sites, which is actually associated with the BAS of the catalysts. Thus, it is realized that the decrease of the strong acid sites ascribed to BAS is mainly due to the de-alumination of ZSM-5 zeolite and the exchange of BAS′ acidic proton with Ga species.7 The results are consistent with previous Py-IR results. Moreover, the numbers of strong acid sites over the Ga/HZSM-5 catalysts treated by reduction–oxidation are more than that over the Ga/HZSM-5 catalysts prepared by the previous treatment,13 because the reduction–oxidation causes less de-alumination of the framework and decrease in BAS. | ||
| Fig. 4 NH3-TPD profiles of different catalysts. (a) HZSM-5; (b) Ga/IM; (c) Ga/IMRO1C; and (d) Ga/IMRO3C. | ||
3.5 H2-TPR
H2-TPR profiles of the as-prepared samples are depicted in Fig. 5, which show the Ga species evolution for each sample. The pure Ga2O3 exhibits two reduction peaks at about 507 °C and 771 °C, which are ascribed to small Ga2O3 particles and segregated bulk Ga2O3, respectively,3 whereas Ga/IM shows three reduction peaks centred at about 528 °C, 671 °C and 810 °C, which are assigned to the small Ga2O3 particles, highly dispersed gallyl ion species probably (GaO)+ and segregated bulk Ga2O3 according to previous research.3,9,21,28,29 In the case of Ga/IMRO1C and Ga/IMRO3C, there are only two reduction peaks observed (centered at about 514–522 °C and 633–692 °C) and the peak ascribed to the segregated bulk Ga2O3 tends to disappear. It is thought that the segregated bulk Ga2O3 can be disassembled into smaller Ga2O3 particles on the ZSM-5 zeolite host as well as formation of well-dispersion gallyl ion species via the reduction–oxidation treatment. Through fitting the TPR profiles and calculating their peak areas (Table S2†), it is found that the (GaO)+ content of Ga/IMRO1C and Ga/IMRO3C are higher than that of the Ga/IM, implying that the reduction–oxidation treatment promotes the formation of highly dispersed (GaO)+ species. This is further verified by XPS results. From the characterization of XPS (Fig. S3†), it is noted that the Ga2p3/2 binding energy (BEs) of Ga/IM (1119.1 eV) is higher than that for Ga2O3 (1117.9 eV), indicating a strong interaction between the ionized Ga species and ZSM-5 zeolite. However, Ga2p3/2 BEs for the Ga/IMRO3C ((1118.6 eV)) and Ga/IMRO1C ((1118.8 eV)) are lower than that for Ga/IM. This is because the reduction–oxidation treatment to Ga/IM promotes the formation of (GaO)+ species easily and induces strong covalent-character bonding between the highly dispersed (GaO)+ species and the framework of the ZSM-5.3 It has also been proved that highly dispersed (GaO)+ species could exchange acidic protons of BAS of the zeolite framework, contributing to the strong LAS.11 The results are concordant with the Py-FTIR results that there are more strong LAS on Ga/IMRO1C and Ga/IMRO3C.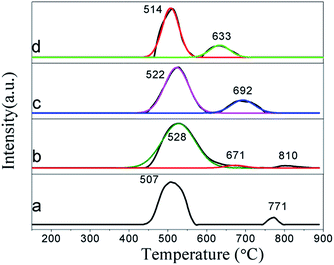 | ||
| Fig. 5 H2-TPR profiles of different samples. (a) Ga2O3; (b) Ga/IM; (c) Ga/IMRO1C; and (d) Ga/IMRO3C. | ||
3.6 TPSR experiment
TPSR is an important technique to study the surface reaction mechanisms of supported metal catalysts,30–36 because it can monitor the evolution of species instantaneously and obtain lots of visualized information during reactions directly. Therefore, in this research, DRIFTS-TPSR and MS-TPSR are used to investigate the mechanistic details in the propane aromatization reaction.In combination with these DRIFT, FTIR, XPS, and NH3-TPD data, we pay more attention to study systematically how acidity of Ga/HZSM-5 catalysts affect the evolution of reactants, intermediates and products on the surface of Ga/HZSM-5 as well as the sorption/desorption process by means of DRIFTS-TPSR and MS-TPSR.
In addition, it is difficult to detect the characteristic peak of GaO+ species by infrared spectroscopy even using probe molecules such as carbon monoxide;10 however, the scientific recognition that highly dispersed (GaO)+ species are present on Ga/IMRO1C and Ga/IMRO3C has been verified as a result of the impregnated incorporation of Ga and subsequent reduction–oxidation treatment, inferred from previous Py-FTIR, H2-TPR and XPS results. As thought,11 the (GaO)+ species can exchange with acidic protons of BAS, generating strong LAS. We deduce that the strong LAS should be the sorption centers for propane and intermediates based on the strong sorption of propane and intermediates on the strong acid sites as shown above.
Conclusively, the above results indicate that the sorption of propane and intermediates mainly occurs on acid sites, especially on strong acid sites. Moreover, the generated strong LAS related to highly dispersed Ga species and BAS on ZSM-5 zeolites have a synergistic effect on sorption/desorption processes of propane and its intermediates.
 | ||
| Fig. 7 (a–d) Propane-TPSR profiles for the as-prepared catalysts. (a) HZSM-5; (b) Ga/IM; (c) Ga/IM RO1C; and (d) Ga/IMRO3C. | ||
Propane aromatization belongs to a tandem process and contains a series of steps such as dehydrogenation and β-scission of propane to propylene and ethylene, oligomerization and interconversion of olefins, olefin alkylation, cyclization and aromatization.4,27 Herein, each step during the reaction is affected by the acidity of catalysts. The DRIFTS-TPSR results have suggested that the sorption of propane and intermediates mainly occurs on acid sites such as BAS related to the Si(OH)Al group and LAS associated with the Al–OH group over ZSM-5 zeolites. Furthermore, previous studies4,27 also showed that the BAS over ZSM-5 zeolite were the exclusive active sites for propane aromatization on pure HZSM-5, contributing to the whole processes of propane aromatization, whereas LAS over ZSM-5 zeolites were only sorption centers. In the Py-FTIR results in Fig. 2D, we observed that the ratios of Brønsted acid concentration/Lewis acid concentration for HZSM-5 are greater than 6.0 at all evacuation temperatures, while the ratio of weak acid site/strong acid site is 1.2 (Table S1†). This indicates that BAS over ZSM-5 zeolite are comprised of weak and strong BAS, which probably corresponds to two types of active sites as mentioned above from the MS-TPSR results. It is thought that both the dehydrogenation of propane to propylene and the β-scission of propane to methane and ethylene occur on the weak and strong BAS over HZSM-5 at low temperatures. With the elevation of reaction temperature, parts of generated propylene and ethylene on strong BAS are further converted into BTX aromatics. In our present catalytic system, the acidic properties of catalyst actually have a remarkably close relation with Ga incorporation. Introduction of Ga to ZSM-5 zeolite can lead to a modest decrease in the number of BAS, which is caused by the exchange of BAS′ acidic protons with Ga species and de-alumination of ZSM-5 zeolite, and the generation of more LAS related to Ga species. As for Ga/IM, the addition of Ga3+ ions into the ZSM-5 inter-crystalline structure is very difficult because of the highly positive electrostatic charge and the bulky size of [Ga(H2O)6]3+ aqua complexes, and thus Ga3+ ions tend to reside predominantly on the external surface of zeolite as single extra-crystalline Ga2O3.42 It has been verified that the extra-crystalline Ga2O3 species have relatively low activity in propane aromatization because their activation is remarkably complicated and difficult. Al-Yassir and Rane et al.2,10 thought that the active species were formed through two main processes: in situ reduction of extra-crystalline Ga2O3 to the highly mobile Ga2O by H2 or hydrocarbons in the feed and subsequent interaction with BAS to form a Z–Ga+ structure (Z− represents an ion-exchange site at the zeolite framework), as shown in eqn (3) and (4).
| Ga2O3 + 2H2 → Ga2O + 2H2O | (3) |
| Ga2O + 2ZH+ → 2Z–Ga+ + H2O | (4) |
Furthermore, the Z–Ga+ structure can catalyse propane to propylene as well as subsequent aromatization via dehydrogenation (as shown in eqn (5) and (6)).10
| Z–Ga+ + C3H8 → Z–Ga+H(C3H7) | (5) |
| Z–Ga+H(C3H7) → Z–Ga+ + H2 + C3H6 | (6) |
In the case of Ga/IMRO1C and Ga/IMRO3C, activation of Ga species proceeds relatively facilely. The reduction–oxidation treatment promotes the formation of these highly dispersed Ga species, probably (GaO)+. After exchanging with acidic protons of BAS, the (GaO)+ species are anchored to ZSM-5 zeolite to form the structures of Z–GaO+, which are ascribed to strong LAS. Finally, the Z–Ga+ structure is easily obtained via deoxygenation of Z–GaO+ in hydrocarbons or H2 flow (as show in eqn (7)).10
| Z–GaO+ + H2 → Z–Ga+ + H2O | (7) |
Therefore, it is apparent that the generated strong LAS related to highly dispersed Ga species are active centers for propane and intermediates on Ga/IMRO1C and Ga/IMRO3C for the aromatization reaction. According to a previous study,11 these strong LAS play a key part in the dehydrogenation process, whereas BAS are responsible for the whole aromatization process. Over Ga/IMRO1C and Ga/IMRO3C, dehydrogenation of propane to propylene occurs on the BAS and strong LAS at low temperatures in the propane-TPSR experiment, whereas β-scission of propane to methane and ethylene only occurs on BAS. With elevation of temperature, the generated propylene and ethylene are further converted into BTX aromatics with release of hydrogen on strong BAS and strong LAS. Based on present TPSR results and our discussion, a new and main reaction pathway for propane transformation is well designed, as shown in Scheme 1.
Apart from that, the incorporation of Ga adds a new dehydrogenation route in propane aromatization and improves dehydrogenation ability of the Ga-containing ZSM-5 zeolites inferred from the above discussions.4 This is further verified by the MS-TPSR results that the peak intensity of hydrogen over Ga-containing ZSM-5 zeolites is obviously enhanced in region II and III with Ga incorporation to HZSM-5. Furthermore, the reduction–oxidation treatment to Ga/IM promotes the formation of strong LAS, which are probably responsible for the generation of the additional θ dehydrogenation peak on Ga/IMRO1C and Ga/IMRO3C. Furthermore, the strong LAS could enhance dehydrogenation steps tremendously in the propane aromatization reaction, including the dehydrogenation of propane to propylene and the conversion of propylene and ethylene to BTX aromatics. It is concluded that the synergistic effect between strong LAS and BAS on Ga/IMRO1C and Ga/IMRO3C contribute to dehydrogenation and β-scission of propane as well as the subsequent aromatization reaction process. It is the synergistic reaction between these two types of acid sites that results in lower aromatization temperature and larger amounts of product of benzene (β state) on Ga/IMRO1C and Ga/IMRO3C than that over Ga/IM. That may be the reason why Ga/IMRO1C and Ga/IMRO3C show higher catalytic performance for propane aromatization than HZSM-5 and Ga/IM, as shown in Fig. 3.
4. Conclusion
The Ga-modified HZSM-5 precursors (Ga/IM), containing 1 wt% Ga, was prepared by the incipient wetness impregnation method, and then subjected to one or three-times treatment of reduction in hydrogen and re-oxidation in air. The resulting Ga/IMRO1C and Ga/IMRO3C catalysts were characterized by techniques, such as N2 physical adsorption, ICP-AES, DRIFT, Py-FTIR, NH3-TPD, H2-TPR, XPS, DRIFT-TPSR and MS-TPSR, to understand the correlations between acidity of these catalysts and their activity for propane aromatization. The characterization data suggested that the impregnated introduction of Ga to ZSM-5 zeolite and subsequent reduction–oxidation treatment led to a great decrease in the numbers of BAS because of the exchange of BAS′ acidic protons with Ga species as well as the severe de-alumination of the ZSM-5 framework, and thus facilely promoted the formation of strong LAS attributed to highly dispersed Ga species, probably (GaO)+, over Ga/IMRO1C and Ga/IMRO3C in comparison with that over Ga/IM. On the other hand, both the BAS and strong LAS were the sorption centers and reaction centers for propane and its intermediates. The strong LAS were experts in promoting the dehydrogenation steps during propane aromatization, whereas the BAS are responsible for the whole aromatization process. According to the TPSR results, the dehydrogenation of propane to propylene occurs on the BAS and strong LAS, whereas β-scission of propane to methane and ethylene only occurs on the BAS at low temperature. With the elevation of temperature, part of the generated propylene and ethylene on the strong BAS and strong LAS were further converted into BTX aromatics with release of hydrogen. It was plausible that the synergistic effect between BAS and strong LAS contributed to the lower aromatization temperature of propane into BTX and more product of benzene (β state) over Ga/IMRO1C and Ga/IMRO3C than that over the Ga/IM.Acknowledgements
This research was supported by the Chinese Academy of Sciences Knowledge Innovation Project (No. KGCX2-YW-318-1), and the Special-funded Program on National Key Scientific Instruments and Equipment Development of China (No. 2011YQ120039).Notes and references
- K. Otto, 22nd World LP Gas Forum, Rio de Janeiro, Brazil, October 8th 2009 Search PubMed
.
- N. Al-Yassir, M. N. Akhtar, K. Ogunronbi and S. Al-Khattaf, J. Mol. Catal. A: Chem., 2012, 360, 1–15 CrossRef CAS
.
- I. Nowak, J. Quartararo, E. G. Derouane and J. C. Védrine, Appl. Catal., A, 2003, 251, 107–120 CrossRef CAS
.
- A. Bhan and W. Nicholas Delgass, Catal. Rev.: Sci. Eng., 2008, 50, 19–151 CrossRef CAS
.
- V. R. Choudhary, A. K. Kinage and T. V. Choudhary, Chem. Commun., 1996, 2545–2546 RSC
.
- R. Fricke, H. Kosslick, G. Lischke and M. Richter, Chem. Rev., 2000, 100, 2303–2405 CrossRef CAS PubMed
.
- M. García-Sánchez, P. C. M. M. Magusin, E. J. M. Hensen, P. C. Thüne, X. Rozanska and R. A. van Santen, J. Catal., 2003, 219, 352–361 CrossRef
.
- G. Krishnamurthy, A. Bhan and W. N. Delgass, J. Catal., 2010, 271, 370–385 CrossRef CAS
.
- B. S. Kwak and W. M. H. Sachtler, J. Catal., 1993, 141, 729–732 CrossRef CAS
.
- N. Rane, A. R. Overweg, V. B. Kazansky, R. A. van Santen and E. J. M. Hensen, J. Catal., 2006, 239, 478–485 CrossRef CAS
.
- V. D. Rodrigues, J. G. Eon and A. C. Faro, J. Phys. Chem. C, 2010, 114, 4557–4567 CrossRef CAS
.
- V. R. Choudhary, K. Mantri and C. Sivadinarayana, Microporous Mesoporous Mater., 2000, 37, 1–8 CrossRef CAS
.
- H. Xiao, J. Zhang, X. Wang, Q. Zhang, H. Xie, Y. Han and Y. Tan, Catal. Sci. Technol., 2015, 5, 4081–4090 Search PubMed
.
- T. Choudhary, A. Kinage, S. Banerjee and V. Choudhary, Energy Fuels, 2006, 20, 919–922 CrossRef CAS
.
- A. Ausavasukhi and T. Sooknoi, Appl. Catal., A, 2009, 361, 93–98 CrossRef CAS
.
- N. Al-Yassir, M. N. Akhtar and S. Al-Khattaf, J. Porous Mater., 2011, 19, 943–960 CrossRef
.
- V. R. Choudhary, A. K. Kinage and T. V. Choudhary, Appl. Catal., A, 1997, 162, 239–248 CrossRef CAS
.
- V. R. Choudhary, A. K. Kinage, C. Sivadinarayana, P. Devadas, S. D. Sansare and M. Guisnet, J. Catal., 1996, 158, 34–50 CrossRef CAS
.
- E. M. El-Malki, R. A. van Santen and W. M. H. Sachtler, J. Phys. Chem. B, 1999, 103, 4611–4622 CrossRef CAS
.
- S. B. A. Hamid, E. G. Derouane, P. Meriaudeau and C. Naccache, Catal. Today, 1996, 31, 327–334 CrossRef
.
- B. Zheng, W. Hua, Y. Yue and Z. Gao, J. Catal., 2005, 232, 143–151 CrossRef CAS
.
- C. Otero Areán, A. L. Bellan, M. P. Mentruit, M. R. g. Delgado and G. T. Palomino, Microporous Mesoporous Mater., 2000, 40, 35–42 CrossRef
.
- O. A. Anunziata and L. B. Pierella, Catal. Lett., 1993, 19, 143–151 CrossRef CAS
.
- S. Altwasser, A. Raichle, Y. Traa and J. Weitkamp, Chem. Eng. Technol., 2004, 27, 1262–1265 CrossRef CAS
.
- A. Raichle, S. Moser, Y. Traa, M. Hunger and J. Weitkamp, Catal. Commun., 2001, 2, 23–29 CrossRef CAS
.
- C. Otero Arean, B. Bonelli, G. Turnes Palomino, A. M. Canaleta Safont and E. Garrone, Phys. Chem. Chem. Phys., 2001, 3, 1223–1227 RSC
.
- A. Montes and G. Giannetto, Appl. Catal., A, 2000, 197, 31–39 CrossRef CAS
.
- K. M. Dooley, C. Chang and G. L. Price, Appl. Catal., A, 1992, 84, 17–30 CrossRef CAS
.
- G. D. Meitzner, E. Iglesia, J. E. Baumgartner and E. S. Huang, J. Catal., 1993, 140, 209–225 CrossRef CAS
.
- Y. Y. Ji, W. Z. Li, H. Y. Xu and Y. X. Chen, Catal. Lett., 2001, 71, 45–48 CrossRef CAS
.
- R. Lin, M. F. Luo, Q. Xin and G. Q. Sun, Catal. Lett., 2004, 93, 139–144 CrossRef CAS
.
- D. Chen, Z. Qu, W. Zhang, X. Li, Q. Zhao and Y. Shi, Colloids Surf., A, 2011, 379, 136–142 CrossRef CAS
.
- R. Q. Long and R. T. Yang, J. Catal., 2001, 198, 20–28 CrossRef CAS
.
- D. Ma, Y. Shu, M. Cheng, Y. Xu and X. Bao, J. Catal., 2000, 194, 105–114 CrossRef CAS
.
- C. Zhao and I. Wachs, Catal. Today, 2006, 118, 332–343 CrossRef CAS
.
- X. Yang, Y. Wei, Y. Su and L. Zhou, Fuel Process. Technol., 2010, 91, 1168–1173 CrossRef CAS
.
- V. d. O. Rodrigues and A. C. F. Júnior, Appl. Catal., A, 2012, 435, 68–77 CrossRef
.
- A. Kurbakova, L. Leimes, V. Aleksanyan, L. Golubinskaya, E. Zorina and V. Bregadze, J. Struct. Chem., 1974, 15, 961–969 CrossRef
.
- F. Eder, M. Stockenhuber and J. A. Lercher, Stud. Surf. Sci. Catal., 1995, 97, 495–500 CrossRef CAS
.
- F. Eder and J. A. Lercher, J. Phys. Chem. B, 1997, 101, 1273–1278 CrossRef CAS
.
- F. Eder and J. A. Lercher, Zeolites, 1997, 18, 75–81 CrossRef CAS
.
- E. S. Shpiro, D. P. Shevchenko, O. P. Tkachenko and R. V. Dmitriev, Appl. Catal., A, 1994, 107, 147–164 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S4; Table S1–S6. See DOI: 10.1039/c5ra15227e |
| This journal is © The Royal Society of Chemistry 2015 |




