Altering synthetic fragments to tune the AIE properties and self-assemble grid-like structures of TPE-based oxacalixarenes†
Zhen Wang‡
a,
Hong Cheng‡b,
Tian-Long Zhaia,
Xianggao Meng*c and
Chun Zhang*a
aCollege of Life Science and Technology, National Engineering Research Center for Nanomedicine, Huazhong University of Science and Technology, Wuhan, Hubei 430074, China. E-mail: chunzhang@hust.edu.cn
bAnalytical and Testing Center, Huazhong University of Science and Technology, China
cSchool of Chemistry, Central China Normal University, Wuhan, Hubei 430079, China. E-mail: xianggao_meng@126.com
First published on 4th September 2015
Abstract
TPE-based oxacalixarenes were synthesized by one-pot condensation. Their AIE properties and self-assembled structures in the solid state can be tuned effectively by utilization different building blocks in the synthetic process. Moreover, the oxacalixarene 1a can be assembled into nanoparticles in THF/water and can be used as a detector for nitroaromatic explosives, such as TNP.
Introduction
Oxacalixarenes,1,2 an important class of macrocyclic host molecules, are synthesized by simple nucleophilic aromatic substitution (SNAr) reactions of diphenol compounds. With the characteristics of ready availability and tunable cavities, great efforts have been attracted in recent years and resulted in a wide variety of oxacalixarenes by different synthetic methods including the fragment coupling approach (FCA)3 and one-pot macrocyclic condensation reaction.4 In this field, the introduction of novel building blocks into oxacalixarene scaffolds to replace phenol units has been promoted greatly the advances in oxacalixarene chemistry. For example, large size building blocks, such as naphthalene,5 triptycene6 and terphenylene derivatives2g,2i have been used to synthesize novel oxacalixarenes with larger-sized cavity. Although the utilization of functional building blocks to synthesize fluorescent oxacalixarenes and to tune their photophysical properties is significant in fields of host-guest chemistry and self-assembly, such oxacalixarenes remain largely unexplored.Tetraphenylethylene and its derivatives (TPEs),7 with the well-known aggregation-induced emission (AIE) effect, have attracted increasing interest in photoelectric materials,8 porous materials,9 and chemo/biosensors.10 TPE has been proved a promising building block to construct oxacalixarene. Recently, we synthesized TPE-based oxacalixarene11a and porous oxacalixarene cage11b and found that these oxacalixarenes showed typical aggregation-induced emission (AIE) properties.
Herein, we introduced the different fragments of dicyanopyridines or dinitrobenzenes into TPE-based oxacalixarene scaffolds 1a or 1b (Scheme 1) by SNAr reaction, respectively. In the oxacalixarenes, the AIE properties of TPE could be turned on or turned off by dicyanopyridines or dinitrobenzenes groups. Based on this result, oxacalixarene 1a could efficiently detect nitroaromatic explosives.
Experimental section
General methods
Melting points, taken on an electrothermal melting point apparatus, are uncorrected. 1H NMR and 13C NMR spectra were recorded on a DMX600 NMR. MALDI-TOF mass spectra were obtained on a BIFLEXIII mass spectrometer. UV spectra were recorded on SHIMADZU UV-2041PC spectrometer. Emission spectra were obtained on HITACHI F-4500 spectrometer. The X-ray single crystal data were collected on Bruker Apex II CCD Area Detector. Materials obtained commercially were used without further purification.Synthesis of 1a
Under a dry argon atmosphere, a mixture of TPE 2 (100 mg, 0.28 mmol), 2,6-dichloropyridine-3,5-dicarbonitrile 3a (54 mg, 0.28 mmol) and anhydrous Cs2CO3 (273 mg, 0.84 mmol) in anhydrous DMSO (5 mL) was stirred vigorously at 25 °C for 1 h. The reaction mixture was partitioned between CH2Cl2 (50 mL) and H2O (40 mL), separated, and the aqueous layer was extracted twice with CH2Cl2 (20 mL). The combined organic layer was washed with brine (100 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The combined organics were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether/CH2Cl2, 5/1) to afford 1a as white solid (50 mg, 37%). Yield: 37.1%. Mp > 300 °C. IR (KBr): 2240, 1614, 1561, 1503, 1431, 1334, 1215, 1156 cm−1. 1H NMR (600 MHz, CDCl3): δ 6.81 (d, J = 8.4 Hz, 8H), 7.02 (d, J = 8.4 Hz, 8H), 7.08 (d, J = 6.6 Hz, 8H), 7.12 (t, J = 7.2 Hz, 8H), 7.15 (m, 4H), 8.19 (s, 2H); 13C NMR (CDCl3, 150 MHz) 91.3, 112.8, 120.9, 127.2, 128.1, 131.3, 132.5, 138.3, 141.4, 143.1, 143.5, 149.2, 149.7, 164.1. MALDI-TOF-MS: m/z 978.20 (M+). Anal. calcd for C66H38N6O4: C, 80.97; H, 3.91; N, 8.58; found: C, 81.23; H, 4.05; N, 8.50.Synthesis of 1b
Under a argon atmosphere, a mixture of TPE 2 (100 mg, 0.28 mmol), 1,5-difluoro-2,4-dinitrobenzene 3b (56 mg, 0.28 mmol) and Cs2CO3 (273 mg, 0.84 mmol) were combined in a 50 mL flask. DMSO (10 mL) was added, and then the reaction mixture was stirred at room temperature for 4 h. The mixture was added with water (40 mL), extracted with EtOAc (3 × 50 mL). The combined organics were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether/CH2Cl2, 1/2) to afford 1b as light yellow solid (45 mg, 31%). Yield: 31.0%. Mp > 300 °C. IR (KBr): 1620, 1591, 1530, 1501, 1349, 1293, 1201, 1164. 1H NMR (600 MHz, CDCl3): δ 8.85 (s, 2H), 7.21 (d, J = 7.2 Hz, 4H), 7.18 (t, J = 15 Hz, 8H), 7.11–7.15 (m, 16H), 6.82 (d, J = 12 Hz, 8H), 6.50 (s, 2H). 13C NMR (150 MHz, CDCl3): 156.69, 151.45, 144.03, 143.33, 141.93, 137.18, 133.93, 132.38, 131.22, 128.22, 127.66, 125.80, 120.78, 104.93. MALDI-TOF-MS: m/z 1057.0 (M+). Anal. calcd for C64H40N4O12: C, 72.72; H, 3.81; N, 5.30; found: C, 72.86; H, 3.65; N, 5.54.Crystallographic data for oxacalixarenes
1a·C4H10O (C70H48N6O5): Mr = 1053.14, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.2726(17), b = 15.102(2), c = 15.213(2) Å, α = 90.795(2)°, β = 101.799(2)°, γ = 93.906(2)°, V = 2752.5(7) Å3, Z = 2, ρcalcd = 1.271 g cm−3, μ = 0.081 mm−1, reflections collected 20
, a = 12.2726(17), b = 15.102(2), c = 15.213(2) Å, α = 90.795(2)°, β = 101.799(2)°, γ = 93.906(2)°, V = 2752.5(7) Å3, Z = 2, ρcalcd = 1.271 g cm−3, μ = 0.081 mm−1, reflections collected 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080, data/restraints/parameters 10092/0/732, GOF on F2 1.020, final R1 = 0.0527, wR2 = 0.1335, R indices (all data): R1 = 0.0988, wR2 = 0.1651, largest diff. peak and hole: 0.415 and −0.256 e Å−3, CCDC-1007294. 1b·0.5CH2Cl2·2C4H10O (C72.5H61ClN4O14): Mr = 1247.70, triclinic, space group P
080, data/restraints/parameters 10092/0/732, GOF on F2 1.020, final R1 = 0.0527, wR2 = 0.1335, R indices (all data): R1 = 0.0988, wR2 = 0.1651, largest diff. peak and hole: 0.415 and −0.256 e Å−3, CCDC-1007294. 1b·0.5CH2Cl2·2C4H10O (C72.5H61ClN4O14): Mr = 1247.70, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 14.4472(19), b = 14.816(2), c = 17.342(2) Å, α = 86.480(2)°, β = 68.308(2)°, γ = 66.251(2)°, V = 3140.4(7) Å3, Z = 2, ρcalcd = 1.319 g cm−3, μ = 0.133 mm−1, reflections collected 22
, a = 14.4472(19), b = 14.816(2), c = 17.342(2) Å, α = 86.480(2)°, β = 68.308(2)°, γ = 66.251(2)°, V = 3140.4(7) Å3, Z = 2, ρcalcd = 1.319 g cm−3, μ = 0.133 mm−1, reflections collected 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762, data/restraints/parameters 10
762, data/restraints/parameters 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939/27/842, GOF on F2 1.023, final R1 = 0.0601, wR2 = 0.1585, R indices (all data): R1 = 0.0887, wR2 = 0.1829, largest diff. peak and hole: 1.230 and −0.535 e/Å3, CCDC-1035320.
939/27/842, GOF on F2 1.023, final R1 = 0.0601, wR2 = 0.1585, R indices (all data): R1 = 0.0887, wR2 = 0.1829, largest diff. peak and hole: 1.230 and −0.535 e/Å3, CCDC-1035320.
Results and discussion
Synthesis of the oxacalixarenes 1a and 1b was carried out by one-pot condensation reaction depicted in Scheme 1. The SNAr reactions of dihydroxytetraphenylethylene 2 with 2,6-dichloropyridine-3,5-dicarbonitrile 3a or 1,5-difluoro-2,4-dinitrobenzene 3b in the presence of Cs2CO3 in DMSO at room temperature resulted in the formation of 1a or 1b in yields of 37.1% or 31.0%, respectively. The products were characterized by IR, 1H NMR, 13C NMR, MALDI-TOF MS, and elemental analysis.12The TPE-based oxacalixarenes 1a and 1b displayed good solubility in common organic solvents. 1H and 13C NMR spectra was used to investigate the conformation of macrocycles 1a and 1b in solution. For 1a, the 1H NMR spectrum in CDCl3 (Fig. S1†) shows only one singlet at 8.19 ppm for the proton Ha of the pyridine moiety. For 1b, the low-field chemical shift of 8.85 ppm for Ha′ and high-field chemical shift of 6.50 ppm for Hb′ were observed in its 1H NMR spectrum in CDCl3 (Fig. S3†). Meanwhile, their 13C NMR spectra both showed fourteen signals for the carbons. These results with only one set of proton and carbon signals in their of NMR experiments indicates that oxacalixarenes 1a and 1b are in fixed conformational structure of 1,3-alternation like our previous results.11a
The structures of oxacalixarenes 1a and 1b were confirmed by their analysis of X-ray single crystal diffraction. The single crystals of oxacalixarenes 1a and 1b suitable for X-ray diffraction were obtained from vapor diffusion of Et2O into dichloromethane solution. Like the conformational structures of most literature-documented oxacalixarenes, oxacalixarene 1a and 1b both adopt 1,3-alternate conformation in the solid state. In the crystal structure of 1a, the four bridging oxygen atoms are located in one plane. The two pyridine rings are eclipsed with a dihedral angle of 81.20°. The transannular N1⋯N4 distance for lower and C3⋯C36 distance for upper rim are 9.353 and 13.614 Å, respectively (Fig. 1a and b). For 1b, the two dinitrobenzene rings are twisted with a dihedral angle of 64.82°. The transannular C6⋯C34 distance for lower and C3⋯C37 distance for upper rim are 9.406 and 14.269 Å, respectively (Fig. 1c and d).
 | ||
| Fig. 1 Top views (a and c) and side views (b and d) of crystal structures of 1a and 1b, respectively. Hydrogen atoms and solvent molecule were omitted for clarity. | ||
In the solid state, the oxacalixarene 1a can assemble into supramolecular grid structure, in which two molecules of Et2O are contained (Fig. 2a). By virtue of a couple of C–H⋯N interactions (dH⋯N = 2.725 Å, θC–H⋯N = 155.43°) and a couple of C–N⋯π interactions (dN⋯π = 3.022 Å) between the cyanopyridine moieties, a dimer structure of 1a was formed (Fig. S7a†). The dimer structure of 1a could be assembled into one-dimensional linear grid structure by a couple of π–π stacking interactions (dπ–π = 3.320 Å) (Fig. S7b†). Different from 1a, the oxacalixarene 1b assembled into two different kinds of grid-like pores structure. Two molecules of 1b with different directions could form a dimer structure by two couples of C–H⋯O interactions (dH⋯O = 2.566 and 2.553 Å, θC–H⋯O = 121.75 and 143.90°) between the aromatic protons of TPE moiety and the oxygen atoms of the dinitrobenzene (Fig. S8a†). The oxacalixarenes could assemble into one-dimensional linear grid structure by another a couple of C–H⋯O interactions (dH⋯O = 2.546 Å, θC–H⋯O = 137.43°) and a couple of π–π stacking interactions (dπ–π = 3.373 Å) between the adjacent dimers (Fig. S8b and c†). The grid-like pore A can be recognized as formation from the four dinitrobenzene moieties. Furthermore, another grid-like pores (pore B) were obtained by two couple of C–H⋯π interactions (dH⋯π = 2.603 Å) between the TPE moieties in the adjacent two grid structures (Fig. S9a and b†). The pore B was formed from four TPE moieties. Due to the different structure and properties, one disordered CH2Cl2 molecule and two molecular ethyl ethers were located in pore A and pore B, respectively (Fig. 2b).
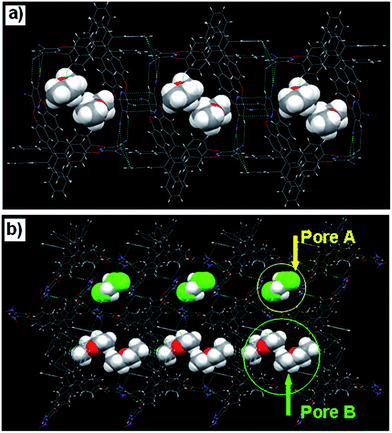 | ||
| Fig. 2 The grid-like structure assembled from oxacalixarene 1a (a), and grid-like structure bearing pore A and pore B assembled from oxacalixarene 1b (b). | ||
The oxacalixarene 1a shows typical AIE properties. In THF solution, 1a is almost non-fluorescent. While, addition of water into the THF solution might “turns on” the fluorescence of 1a because of the AIE effect. As shown in Fig. 3a, the fluorescence intensity of 1a is significantly enhanced when the volume ratio of water/THF is larger than 70/30, which can be detected by naked eye (Fig. 3b). From the dynamic light scattering experiment (Fig. S10a†) and transmission electron micrograph (TEM) results (Fig. S10b†), 1a can be assembled into nanoparticles with size of ∼100 nm in water/THF (95/5). Different from 1a, the AIE effect of TPE was turned off by introducing the dinitrobenzenes into oxacalixarene 1b, which might result from photoinduced electron transfer between the TPE and dinitrobenzene units.
Inspired by the results of fluorescence quenching of TPE in 1b, oxacalixarene 1a can be used as a detector for nitroaromatic explosives, which is significant for national security and environmental protection. The recognition of 2,4,6-trinitrophenol (TNP) was studied by fluorescent titration experiments. As shown in Fig. 4a, the emission of 1a in water/THF (95/5) was quenched upon addition of TNP. The quenching constant of 1a for TNP was determined from the exponential quenching equation (I/I0 = AeK[Q] + B).13 By the nonlinear curve-fitting, the quenching constant of 1a with TNP was calculated to 1.7 × 104 M −1.
To investigate the selectivity of 1a for nitroaromatic explosives, nine aromatic compounds were used as quenchers, including TNP, 2,4-dinitrophenol (DNP), p-nitrophenol (p-NP), o-nitrophenol (o-NP), m-nitrophenol (m-NP), o-nitrotoluene (NT), 4-nitrobenzoic acid (NBA), benzoic acid (BA) and phenol (PH). When 15 equiv. of quenchers were added into the suspension of 1a in water/THF (95/5), the fluorescent intensities were measured (Fig. S11–S19†).12 As shown in Fig. 4b, 1a displayed greater fluorescence quenching with electron deficient nitroaromatic compounds than with other aromatic compounds. Among these electron deficient nitroaromatic compounds, TNP induced the greatest quenching with the most electron-deficient characteristic. The quenching efficiency ((1 − I/I0) × 100%) of TNP is up to 74.3%. The quenching constant of 1a with TNP is also the greatest in the nine aromatic compounds (Table S1†). These results suggested that the greater the number of nitro-groups present on the aromatic ring, the more extensive the degree of fluorescence quenching. In order to determine the limits of detection of TNP by 1a, the fluorescence changes of 1a (5.0 × 10−7 M) with different amounts of TNP were measured as shown in Fig. S20.† By this method, the sensitivity of 1a toward TNP was down to 0.1 μM (23 μg L−1).
Conclusions
In conclusion, we have synthesized TPE-based oxacalixarenes by the SNAr reactions. Their AIE properties can be tuned effectively by different building blocks such as cyanopyridine or nitrobenzene. With typical AIE properties, the oxacalixarene 1a shows good detection abilities towards nitroaromatic explosives such as TNP. Further work on these expanded oxacalixarenes will be focused on their co-assembly and recognition of some interesting guests, which are now underway in our lab.Acknowledgements
This work is supported by the National Natural Science Foundation of China (20902031, 21272173 and J1103514), and the Fundamental Research Funds for the Central Universities (HUST 2015TS086). We thank the Analytical and Testing Center of Huazhong University of Science and Technology for related analysis. We also thank Dr Yang Yong in Zhejiang Sci-Tech Univ. for 1,5-difluoro-2,4-dinitrobenzene.Notes and references
- For recent reviews, see: (a) M.-X. Wang, Chem. Commun., 2008, 4541 RSC; (b) W. Maes and W. Dehaen, Chem. Soc. Rev., 2008, 37, 2393 RSC; (c) M.-X. Wang, Acc. Chem. Res., 2012, 45, 182 CrossRef CAS PubMed.
- For recent examples of oxacalixarenes, see: (a) H. Zhang, B. Yao, L. Zhao, D.-X. Wang, B.-Q. Xu and M.-X. Wang, J. Am. Chem. Soc., 2014, 136, 6326 CrossRef CAS; (b) D.-X. Wang and M.-X. Wang, J. Am. Chem. Soc., 2013, 135, 892 CrossRef CAS PubMed; (c) T. Xie, S.-Z. Hu and C.-F. Chen, J. Org. Chem., 2013, 78, 981 CrossRef CAS; (d) C. Yang, Y. Chen, D.-X. Wang, L. Zhao and M.-X. Wang, Org. Lett., 2013, 15, 4414 CrossRef CAS PubMed; (e) Y. Visitaev, I. Goldberg and A. Vigalok, Inorg. Chem., 2013, 52, 6779 CrossRef CAS PubMed; (f) S. Li, S.-X. Fa, Q.-Q. Wang, D.-X. Wang and M.-X. Wang, J. Org. Chem., 2012, 77, 1860 CrossRef CAS; (g) W.-J. Hu, X.-L. Zhao, M.-L. Ma, F. Guo, X.-Q. Mi, B. Jiang and K. Wen, Eur. J. Org. Chem., 2012, 1448 CrossRef CAS PubMed; (h) N. P. Bizier, J. P. Vernamonti and J. L. Katz, Eur. J. Org. Chem., 2012, 2303 CrossRef CAS PubMed; (i) M. M. Naseer, D.-X. Wang and M.-X. Wang, Heterocycles, 2012, 84, 1375 CrossRef CAS; (j) D.-X. Wang, S.-X. Fa, Y. Liu, B.-Y. Hou and M.-X. Wang, Chem. Commun., 2012, 48, 11458 RSC; (k) W. Van Rossom, J. Caers, K. Robeyns, L. Van Meervelt, W. Maes and W. Dehaen, J. Org. Chem., 2012, 77, 2791 CrossRef CAS PubMed; (l) Y.-P. Zhu, J.-J. Yuan, Y.-T. Li, M. Gao, L.-P. Cao, J.-Y. Ding and A.-X. Wu, Synlett, 2011, 52 Search PubMed; (m) Y. Chen, D.-X. Wang, Z. T. Huang and M.-X. Wang, Chem. Commun., 2011, 47, 8112 RSC; (n) W. Van Rossom, O. Kundrat, T. H. Ngo, P. Lhotak, W. Dehaen and W. Maes, Tetrahedron Lett., 2010, 51, 2423 CrossRef CAS PubMed; (o) W. Van Rossom, L. Kishore, K. Robeyns, L. Van Meervelt, W. Dehaen and W. Maes, Eur. J. Org. Chem., 2010, 4122 CrossRef CAS PubMed; (p) M. E. Alberto, G. Mazzone, N. Russo and E. Sicilia, Chem. Commun., 2010, 46, 5894 RSC; (q) X.-Y. Li, L.-Q. Liu, M.-L. Ma, X.-L. Zhao and K. Wen, Dalton Trans., 2010, 39, 8646 RSC.
- M.-X. Wang and H.-B. Yang, J. Am. Chem. Soc., 2004, 126, 15412 CrossRef CAS.
- J. L. Katz, M. B. Feldman and R. R. Conry, Org. Lett., 2005, 7, 91 CrossRef CAS PubMed.
- J. L. Katz, B. J. Geller and P. D. Foster, Chem. Commun., 2007, 1026 RSC.
- (a) C.-F. Chen, Chem. Commun., 2011, 47, 1674 RSC; (b) S.-Z. Hu and C.-F. Chen, Chem. Commun., 2010, 46, 4199 RSC; (c) C. Zhang and C.-F. Chen, J. Org. Chem., 2007, 72, 3880 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- (a) Q. Chen, D.-Q. Zhang, G.-X. Zhang, X.-Y. Yang, Y. Feng, Q.-H. Fan and D.-B. Zhu, Adv. Funct. Mater., 2010, 20, 3244 CrossRef CAS PubMed; (b) C. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 62 CrossRef CAS PubMed; (c) Z. Wang, S. Chen, J. W. Y. Lam, W. Qin, R. T. K. Kwok, N. Xie, Q. Hu and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 8238 CrossRef CAS; (d) G. Huang, B. Ma, J. Chen, Q. Peng, G.-X. Zhang, Q. Fan and D.-Q. Zhang, Chem.–Eur. J., 2012, 18, 3886 CrossRef CAS PubMed; (e) X.-G. Gu, J.-J. Yao, G.-X. Zhang, C. Zhang, Y.-L. Yan, Y.-S. Zhao and D.-Q. Zhang, Chem.–Asian J., 2013, 8, 2362 CrossRef CAS PubMed; (f) X.-Q. Zhang, Z.-G. Chi, H.-Y. Li, B.-J. Xu, X.-F. Li, W. Zhou, S.-W. Liu, Y. Zhang and J.-R. Xu, Chem.–Asian J., 2011, 6, 808 CrossRef CAS; (g) B.-J. Xu, Z.-G. Chi, H.-Y. Li, X.-Q. Zhang, X.-F. Li, S.-W. Liu, Y. Zhang and J. R. Xu, J. Phys. Chem. C, 2011, 115, 17574 CrossRef CAS; (h) Z.-J. Zhao, S.-M. Chen, J. W. Y. Lam, P. Lu, Y.-C. Zhong, K.-S. Wong, H.-S. Kwok and B.-Z. Tang, Chem. Commun., 2010, 46, 2221 RSC.
- (a) Y. Xu, L. Chen, Z. Guo, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2011, 133, 17622 CrossRef CAS; (b) Q. Chen, J.-X. Wang, F. Yang, D. Zhou, N. Bian, X.-J. Zhang, C.-G. Yan and B.-H. Han, J. Mater. Chem., 2011, 21, 13554 RSC; (c) N. B. Shustova, B. D. McCarthy and M. Dincă, J. Am. Chem. Soc., 2011, 133, 20126 CrossRef CAS PubMed.
- (a) M. Wang, G.-X. Zhang, D.-Q. Zhang, D.-B. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858 RSC; (b) Y. Liu, C. M. Deng, L. Tang, A.-J. Qin, R.-R. Hu, J.-Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2011, 133, 660 CrossRef CAS PubMed; (c) M. Wang, X.-G. Gu, G.-X. Zhang, D.-Q. Zhang and D.-B. Zhu, Anal. Chem., 2009, 81, 4444 CrossRef CAS PubMed; (d) X.-G. Gu, G.-X. Zhang and D.-Q. Zhang, Analyst, 2012, 137, 365–369 RSC; (e) X.-G. Gu, G.-X. Zhang, Z. Wang, W.-W. Liu, L. Xiao and D.-Q. Zhang, Analyst, 2013, 138, 2427 RSC; (f) T. Sanji, K. Shiraishi, M. Nakamura and M. Tanaka, Chem.–Asian J., 2010, 5, 817 CrossRef CAS PubMed; (g) X.-M. Hu, Q. Chen, J.-X. Wang, Q.-Y. Cheng, C.-G. Yan, J. Cao, Y.-J. He and B.-H. Han, Chem.–Asian J., 2011, 6, 2376 CrossRef CAS PubMed; (h) N.-N. Liu, S. Song, D.-M. Li and Y.-S. Zheng, Chem. Commun., 2012, 48, 4908 RSC; (i) H.-T. Feng and Y.-S. Zheng, Chem.–Eur. J., 2014, 20, 195 CrossRef CAS PubMed; (j) H.-T. Feng and Y.-S. Zheng, J. Mater. Chem. C, 2014, 2, 2353 RSC.
- (a) C. Zhang, Z. Wang, S. Song, Y.-S. Zheng, X.-L. Yang and H.-B. Xu, J. Org. Chem., 2014, 79, 2729 CrossRef CAS PubMed; (b) C. Zhang, Z. Wang, L. Tan, T.-L. Zhai, S. Wang, B. Tan, Y.-S. Zheng, X.-L. Yang and H.-B. Xu, Angew. Chem., Int. Ed., 2015, 54, 9244–9248 CrossRef CAS PubMed.
- See ESI†.
- J.-Z. Liu, Y.-C. Zhong, P. Lu, Y.-N. Hong, J. W. Y. Lam, M. Faisal, Y. Yu, K. S. Wong and B.-Z. Tang, Polym. Chem., 2010, 1, 426 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1007294 and 1035320. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra15214c |
| ‡ The authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |



