Highly selective thin film composite hollow fiber membranes for mixed vapor/gas separation†
Abstract
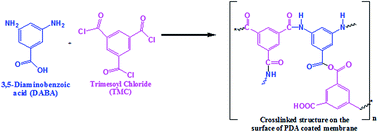
In the present study, thin film composite membranes have been prepared using an interfacial polymerization method. First, we coated a polydopamine (PDA) layer using different concentrations of PDA solution on polyethersulfone (PES) hollow fiber supports. After the PDA coating layer, thin film composite (TFC) membranes were prepared with 3,5-diaminobenzoic acid (3,5-DABA) as an aqueous phase monomer and trimesoyl chloride (TMC) as an organic phase monomer to synthesize a hydrophilic polyamide layer. This prepared selective layer is considered desirable to fabricate hydrophilic TFC membranes for water vapor/N2 separation. The TFC membranes by interfacial polymerization were confirmed and discussed using the accumulated results of characterization. Hollow fiber membranes (HFM) surface modification with PDA before TFC coatings was proposed to modestly and effectively enhance the membrane selectivity. The newly prepared TFC hollow fiber membranes acquired reasonably excellent selectivity and superior permeation fluxes. As a result, membrane sample MS4 coated with 2.0 wt% PDA and TFC prepared using 0.5 wt% of 3,5-DABA with 0.2 wt% of TMC and 60 s of reaction time showed the best permeance and selectivity as 3185 GPU and 195, respectively, compared with other TFC membranes prepared with different PDA concentrations. Overall, the membranes showed good performance in the entire range of operating conditions investigated.

 Please wait while we load your content...
Please wait while we load your content...