Microwave assisted synthesis of high-surface area WO3 particles decorated with mosaic patterns via hydrochloric acid treatment of Bi2W2O9†
Abstract
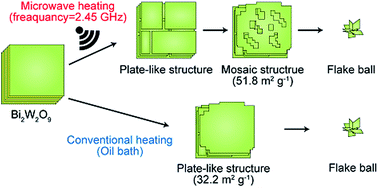
Monoclinic WO3 particles with mosaic structures on the planes of the particles were synthesized from layered bismuth tungstate (Bi2W2O9) with the alternate stacked structure of Bi2O22+ layers and W2O72− layers via a hydrothermal technique using hydrochloric acid at 200 °C under microwave heating. These particles possessed high surface areas, giving a high photocatalytic activity in the degradation of gaseous acetaldehyde. Sequential SEM observations have clarified the dynamic transformations of the structures of Bi2W2O9 under microwave heating in comparison with conventional heating. The WO3 production through the reaction of Bi2W2O9 with HCl consists of two reaction steps, i.e., H2W2O7 generation via replacement of Bi2O22+ with H+ (the first step) and conversion of H2W2O7 to WO3 through dehydration of H2W2O7 (the second step). The first step proceeds even at room temperature, while the second reaction requires temperatures above 180 °C. To investigate the microwave heating effect on the first step (the replacement of Bi2O22+), the reaction of Bi2W2O9 and HCl was carried out at 80 °C under both microwave heating and conventional heating. It has been found that the replacement of Bi2O22+ with H+ is accelerated by the microwave selective heating effect. Interestingly, the WO3 particles with mosaic patterns were produced only under microwave heating. On the other hand, conventional heating of Bi2W2O9 in the presence of HCl resulted in the formation of plate-like WO3 particles without mosaic patterns.


 Please wait while we load your content...
Please wait while we load your content...