N-annelated perylenes as effective green emitters for OLEDs
A. Bučinskasa,
D. Volyniuka,
Y. Danyliva,
J. V. Grazulevicius*a,
G. Baryshnikovb,
B. Minaevb,
K. Ivaniukc,
V. Cherpakc and
P. Stakhirac
aDepartment of Polymer Chemistry and Technology, Department of Organic Technology, Kaunas University of Technology, Radvilenu Plentas 19, LT-50254 Kaunas, Lithuania. E-mail: Juozas.Grazulevicius@ktu.lt; Tel: +370 37300193
bBohdan Khmelnytsky National University, Shevchenko 81, 18031 Cherkassy, Ukraine
cLviv Polytechnic National University, S. Bandera 12, 79013 Lviv, Ukraine
First published on 7th September 2015
Abstract
New carbazole-substituted N-annelated perylenes with different linking topologies of the chromophores were synthesized and their physical properties were studied with various experimental techniques including thermogravimetric analysis, differential scanning calorimetry, UV-vis, fluorescence and photoelectron emission spectroscopies and cyclic voltammetry. The synthesized materials exhibit extremely high thermal stability with 5% weight loss temperatures ranging from 400 to 457 °C. Photoluminescence quantum yields of the solid films of the compounds range from 33 to 51%. The ionization potentials of the solid samples established by the electron photoemission technique were found to be in the range of 5.14–5.53 eV. Using the newly synthesized compounds as emissive materials, efficient organic light-emitting diodes (OLEDs) were fabricated with the emission pattern covering the broad visible region from 475 to 675 nm. Bright green OLEDs exhibited luminance exceeding 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and external quantum efficiency reaching 4.2%.
000 cd m−2 and external quantum efficiency reaching 4.2%.
Introduction
Increasing interest in the application of ambipolar organic semiconductors for fabrication of efficient organic light emitting devices requires balanced charge carrier transport and high solid-state photoluminescence (PL) quantum yields of emitting materials.1–3 Numerous emissive donor–acceptor combinations including bipolar low-molar-mass compounds,4,5 conjugated-polymer blends,6 organic molecules mixtures7–9 and organic–inorganic host–guest systems10–12 have been used for organic light-emitting diodes (OLEDs) fabrication. Perylene derivatives form a class of interesting n-type organic semiconductors owing to excellent charge carrier transport together with the outstanding chemical, thermal and photochemical stability.13,14 Very high electron mobility of perylene derivatives has qualified them to be widely employed in electronic and optoelectronic devices.15,16 Carbazole derivatives have been also extensively studied as the hole-transporting or emitting materials for OLEDs.17,18 The combination of hole-transporting carbazole and with electron-transporting perylene at molecular level may lead to new donor–acceptor molecular materials with interesting and useful optical and charge-transporting properties important for various device applications. In the present work, we describe the first synthetic protocol for the carbazole-substituted N-annelated perylenes which possess the three different types of linkages between carbazole and perylene moieties. The presence of the electron-transporting N-annelated perylene core in the molecules together with the peripheral hole-transporting carbazole moieties allowed us to obtain the excellent balance between the hole and electron mobilities in the thin films. On the basis of experimental studies and quantum chemical calculations in this paper we disclose the impact of linking topology on the thermal, electrochemical optical and photophysical properties of the newly synthesized derivatives of N-annelated perylene and carbazole and demonstrate their applicability for the fabrication of highly efficient OLEDs.Results and discussion
Synthesis
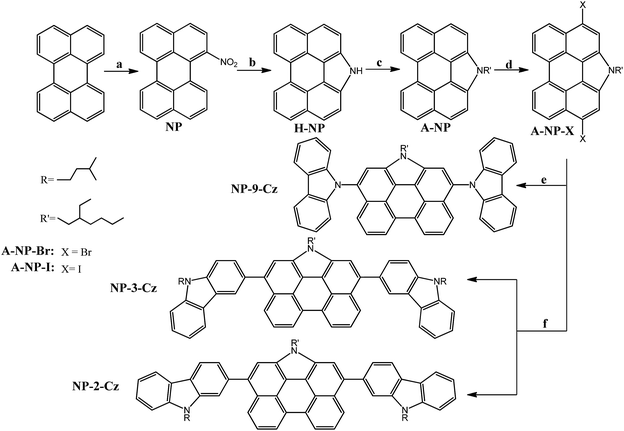
The synthetic route to the target compounds 3,10-di(9H-carbazol-9-yl)-1-(2-ethylhexyl)-1H-phenanthro[1,10,9,8-cdefg]carbazole (NP-9-Cz), 1-(2-ethylhexyl)-3,10-bis(9-isopentyl-9H-carbazol-3-yl)-1H-phenanthro[1,10,9,8-cdefg]carbazole (NP-3-Cz), 1-(2-ethylhexyl)-3,10-bis(9-isopentyl-9H-carbazol-2-yl)-1H-phenanthro[1,10,9,8-cdefg]carbazole (NP-2-Cz) consisting of N-annelated perylene core and the differently linked carbazolyl substituents are shown in Scheme 1. NP-3-Cz, NP-2-Cz were synthesized by the Suzuki–Miyaura coupling reaction9 of 3,10-dibromo-1-(2-ethylhexyl)-1H-phenanthro[1,10,9,8-cdefg]carbazole (A-NP-Br) with 9-isopentyl-3-carbazolyl and 9-isopentyl-2-carbazolyl boronic acid pinacol ester, respectively. NP-9-Cz was obtained by Ullmann-coupling19 of iodinated precursor 3,10-diiodo-1-(2-ethylhexyl)-1H-phenanthro[1,10,9,8-cdefg]carbazole (A-NP-I) and carbazole. Halogenated N-annelated perylene was prepared in four steps including nitration of commercially available perylene31 followed by a Cadogan cyclization using triphenylphosphine,30 alkylation with 1-Br-3-methylbutane20 and bromination reaction with NBS in DMF29 or Tucker iodination.13 The boronic acid pinacol esters of carbazole (3-BCz, 2-BCz) were prepared from 3-bromocarbazole (3Br-Cz) and 2-bromocarbazole (2Br-Cz) by N-alkylation and borylation using the procedure reported in literature.14 The chemical structures of the compounds were confirmed by 1H and 13C NMR and mass spectrometries. The target compounds NP-9-Cz, NP-3-Cz, NP-2-Cz were found to be soluble in common organic solvents such as chloroform, toluene, acetone, tetrahydrofuran.Thermal properties
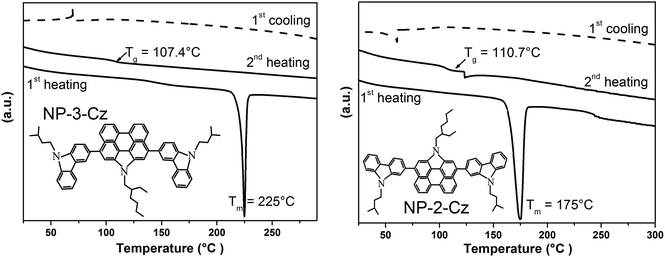
Thermal transitions of NP-3-Cz, NP-9-Cz, NP-2-Cz were investigated by TGA and DSC. The DSC curves of NP-3-Cz and NP-2-Cz are shown in Fig. 1 and the thermal characteristics of all three compounds are collected in Table 1. NP-3-Cz, NP-9-Cz and NP-2-Cz were found to exhibit very high thermal stability. Their 5% weight loss temperatures range from 400 °C to 457 °C. The slightly lower thermal stability of NP-9-Cz can apparently be explained by the fact that the C–N bonds between the carbazole and phenanthrocarbazole moieties are less stable than the corresponding C–C links.The melting points determined from the sharp endothermic peaks in the thermograms of DSC were found to be 225, 241 °C, 175 °C for NP-3-Cz, NP-9-Cz and NP-2-Cz respectively. No exothermic peaks associated with crystallization were observed for compounds NP-3-Cz and NP-2-Cz when their melted samples were cooled down to the room temperature and amorphous solid was formed. When the melted sample of NP-9-Cz was cooled down broad exothermic peak due to crystallization was observed at ca. 215 °C. On the repeated heating cycles the samples of NP-3-Cz and NP-2-Cz showed glass transitions at 107 °C and 111 °C, respectively, and no other signals were observed. Meanwhile, on the second heating scan compound NP-9-Cz demonstrated endothermic melting peak at the same temperature (241 °C) as it was observed on the first heating cycle. Thus it was not possible to transform NP-9-Cz into the glassy state by slow cooling from the melt. The stronger disposition to crystallization of NP-9-Cz having no branched alkyl chains at carbazole moieties may be due to the more favorable arrangement and stacking in the crystal as compared to NP-3-Cz and NP-2-Cz. Nevertheless, thin uniform films of NP-9-Cz could be prepared by solution processing and by vacuum evaporation.
Optical and photophysical properties
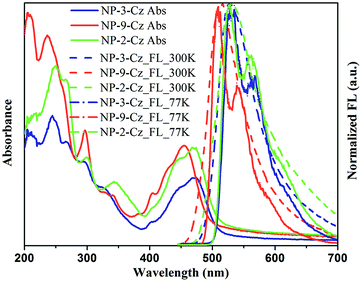
UV and FL spectra of the dilute solutions of NP-3-Cz, NP-9-Cz and NP-2-Cz in THF and of the solid films are shown in Fig. 2 and 3. The optical and photophysical characteristics are summarized in Table 2.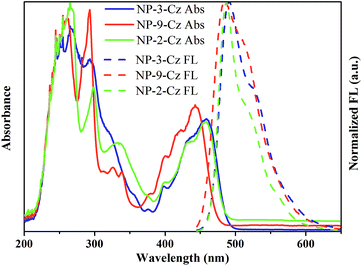 | ||
| Fig. 2 UV-vis and normalized fluorescence spectra (λex = 350 nm) of dilute THF solutions (10−5 M) of NP-3-Cz, NP-9-Cz and NP-2-Cz. | ||
| λabmax/λabmax film, nm | λemmax sol/λemmax film, nm | Stokes shift, nm | Φsol/Φfilm, % | S1a/S1b, eV | T1, eV | fS0S1 | τ, ns | χ2 | krad (108 s−1 ns) | knrad (108 s−1 ns) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Singlet levels of investigated molecules have been taken as the energies of maxima of FL spectra.b Triplet levels of investigated molecules have been calculated using the TD-DFT/B3LYP/6-31G(d) method with the PCM solvent approach (THF was used as the model solvent accordingly to the experimental measurements). | |||||||||||
| NP-3-Cz | 458/472 | 491/528 | 33/56 | 76/33 | 2.56/2.69 | 1.76 | 0.90 | 3.15 | 1.15 | 2.4 | 0.8 |
| NP-9-Cz | 443/455 | 485/514 | 42/59 | 68/51 | 2.54/2.79 | 1.80 | 0.49 | 4.07 | 1.08 | 1.7 | 0.8 |
| NP-2-Cz | 457/471 | 487/528 | 30/57 | 61/42 | 2.53/2.72 | 1.76 | 1.06 | 2.62 | 1.18 | 2.3 | 1.5 |
UV spectra of the dilute solutions and of the solid films of NP-3-Cz and NP-2-Cz have comparable absorption profiles. The absorption bands observed in spectra of the solutions of NP-3-Cz, NP-9-Cz and NP-2-Cz in the region of 285–310 nm can be assigned to nπ* transition of carbazole moiety.21,22 The lowest-energy absorption bands observed at 460 nm in the UV spectra of the solutions of NP-3-Cz and NP-2-Cz are red shifted by ca. 20 nm relative to that of NP-9-Cz. These absorption bands can be attributed to the ππ* transition in the annelated perylene core24 in a good agreement with the quantum-chemical calculations. As it can be seen from Fig. 4 both HOMO and LUMO orbitals are localized on the central 6H-phenanthro[1,10,9,8-cdefg]carbazole core with the small contributions on the peripheral carbazole moieties. It should be noted that the minor charge transfer from the carbazole fragment to the phenanthrocarbazole fragment occurs upon the HOMO–LUMO transition for all the compounds. The share of charge-transfer configuration increases in the series of NP-2-Cz, NP-3-Cz and NP-9-Cz which corresponds to the decreasing of the oscillator strength (fS0S1) of the first singlet–singlet transition. This result corresponds to the lower radiation rate constant for luminescence of NP-9-Cz because of the fS0S1 directly proportional to the krad values in accordance with the following equation: krad = ν2fS0S1/1.5003, where ν is the wavenumber for the corresponding transition S0 → S1 (represented in cm−1). These results provide evidence of more effective conjugation of π-electrons in the molecules of NP-3-Cz and NP-2-Cz compared to that in NP-9-Cz. Dilute solutions of compounds NP-3-Cz, NP-9-Cz and NP-2-Cz show intense green emission with the wavelengths of intensity maxima ranging between 485 and 491 nm and fluorescence quantum yields ranging from 61 to 76%. The emission intensity maxima of the solid samples are red-shifted up to 40 nm and the quantum yield values are lower by 20–40%.
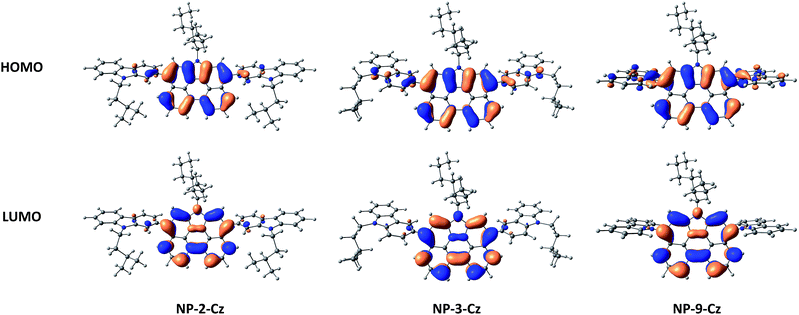 | ||
| Fig. 4 Frontier molecular orbitals of NP-2-Cz, NP-3-Cz and NP-9-Cz stacked dimer calculated with the B3LYP/6-31G(d) method. | ||
We have to stress that the global potential energy curves (PECs) minima of the ground and first excited (S1) singlet states almost coincide because of the small Stocks shift and mirror relationship for absorption and fluorescence spectral contours. Another indirect confirmation of this conclusion comes from our UB3LYP and TD DFT calculation of the excited triplet state (T1) and its comparison with the properties of S0, S1 states. The T1 and S1 states are both produced by HOMO–LUMO excitation, thus their geometrical characteristics are similar. On the other hand, geometry optimization of the T1 structure provides quite similar parameters to the ground state one. Thus, we can conclude, that the calculated oscillator strength values for the first singlet–singlet electronic transition determines directly the intensity of the reverse emissive S1 → S0 transition. This fact allows us to explain the high quantum yields values for NP-3-Cz, NP-9-Cz and NP-2-Cz by the high TD DFT calculated values of fS0S1.
To get deeper insight into the photophysical properties of NP-3-Cz, NP-9-Cz and NP-2-Cz fluorescence decay curves of the solutions were recorded. Fluorescence decay curves were well described by the single-exponential functions for all the target compounds (Fig. 5). Compounds NP-3-Cz, NP-9-Cz and NP-2-Cz showed close values of fluorescence life time (τ) which were found to be 3.15, 4.07 and 2.62 ns, respectively. The radiative (kr) and nonradiative (knr) decay rate constants of the singlet excited state were calculated using the following equations: kr = Φ/τ, knr = (1 − Φ)/τ. The estimated values are summarized in Table 2. Compounds NP-3-Cz and NP-2-Cz exhibited almost the same kr values while knr was found to be by almost 2 times higher for NP-2-Cz. As a consequence of more preferable nonradiative processes in solution of NP-2-Cz this compound showed by ca. 15% lower fluorescence quantum yield as compared to NP-3-Cz. Fig. 3 shows the absorption and emission spectra of the solid films of the target compounds. The lowest-energy absorption band of NP-3-Cz, NP-9-Cz and NP-2-Cz were observed at 528, 514, and 528 nm, respectively. The lowest energy absorption bands of the thin films of NP-3-Cz, NP-9-Cz and NP-2-Cz exhibited red-shifts of ca. 30 nm with respect to those of the dilute solutions. This difference is generally a result of the intermolecular interactions in the solid state.
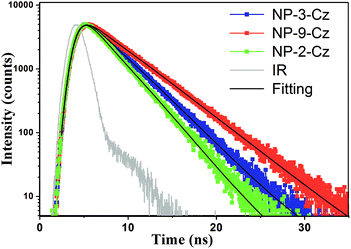 | ||
| Fig. 5 Fluorescence decay curves of the dilute (10−5 M) solutions in THF of NP-3-Cz (blue), NP-9-Cz (red), NP-2-Cz (green). | ||
Electrochemical and photoelectrical properties
The electrochemical properties of NP-9-Cz, NP-3-Cz and NP-2-Cz were investigated by cyclic voltammetry (CV) to analyze energy transfer processes and the reversibility of oxidation–reduction processes. The CV curves of NP-9-Cz, NP-3-Cz and NP-2-Cz are shown in Fig. 6.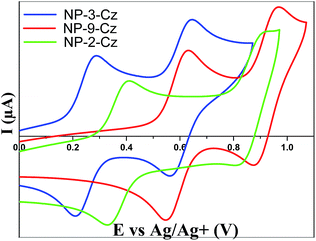 | ||
| Fig. 6 Cyclic voltammograms of NP-9-Cz, NP-3-Cz and NP-2-Cz at 10−3 mol L−1 in a solution of in argon-purged TBAP (0.1 M) in CH2Cl2. v = 50 mV s−1. | ||
The electrochemical characteristics are summarized in Table 2. The cyclic voltammograms of NP-3-Cz, NP-9-Cz and NP-2-Cz exhibited two reversible oxidation waves with the peaks at 0.29, 0.63, 0.41 V (vs. Fc/Fc+) of the first wave and 0.64, 0.97, 0.91 V of the second wave, respectively. The redox processes of the solutions were found to be stable with no obvious changes during subsequently repeated scanning (over 5 cycles). The highest occupied molecular orbital (HOMO) energy levels of NP-3-Cz, NP-9-Cz and NP-2-Cz were estimated to be −4.95, −5.43 and −5.12 eV respectively, according to the relationship EHOMO (eV) = −1.4Eonset,ox (V) − 4.6 (ref. 23) where Eonset,ox is the onset of the first oxidation wave.24 The DFT-calculated HOMO energy levels are in a good agreement with the experimentally estimated values (Table 3), while the theoretical bandgap energies deviate to a greater extent from the experimental values since the calculated E (LUMO) energies are evidently overestimated which is a known problem of DFT.
| Compound | d, [μm] | μh0/μe0a [cm2 V−1 s−1] | μh/μeb [cm2 V−1 s−1] | α × 10−3 [cm V−1] | λec, eV | λhc, eV |
|---|---|---|---|---|---|---|
| a The zero-field hole and electron drift mobility.b Mobility value at an electric field of 2.5 × 105 V cm−1.c The electron (λe) and hole (λh) reorganization energies obtained from the B3LYP/6-31G(d) calculations. | ||||||
| NP-3-Cz | 2.1 | 8.2 × 10−5/9.4 × 10−6 | 9.1 × 10−4/5.4 × 10−5 | 4.9/3.5 | 0.25 | 0.25 |
| NP-9-Cz | 1.0 | 1.4 × 10−5/2.2 × 10−5 | 2.5 × 10−4/3.9 × 10−4 | 5.8/1.4 | 0.22 | 0.37 |
| NP-2-Cz | 4.2 | 6.6 × 10−4/1.1 × 10−3 | 9.3 × 10−3/2.6 × 10−3 | 5.3/1.7 | 0.29 | 0.21 |
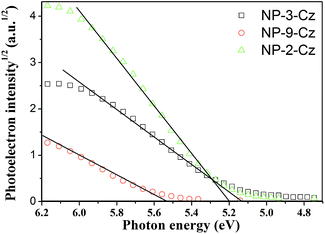
Ionization potentials (IP) of the solid layers of the synthesized compounds were measured by the electron photoemission in air technique. The photoelectron emission spectra of NP-3-Cz, NP-9-Cz and NP-2-Cz are presented in Fig. 7. The IP values were obtained from the intersection of the linear parts of the photoelectron spectra drawn with the abscissa axis. The ionization potential values vary in range from 5.14 to 5.53 eV. The IP value observed for NP-9-Cz was found to be by 0.3 eV higher compared to those of NP-3-Cz and NP-2-Cz.
Charge-transporting properties
To characterize the charge transporting properties the layers of NP-3-Cz, NP-9-Cz and NP-2-Cz were investigated using time-of-flight technique at room temperature in air. The layers of all the three compounds were found to be capable to transport both holes and electrons in air. This finding is in a good agreement with the close values of the DFT calculated electron (λe) and hole (λh) reorganization energies (the higher λ values correspond to the lower parameters of conductivity). Fig. 8 shows electric field dependencies of charge carrier mobilities (μ) for the layers of NP-3-Cz, NP-9-Cz and NP-2-Cz. The linear dependencies of charge mobilities on the square root of the electric field (E1/2) were observed for all the compounds.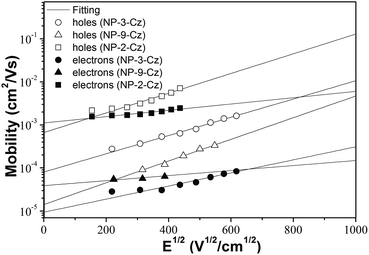 | ||
| Fig. 8 Electric field dependencies of hole and electron mobilities in the layers of NP-3-Cz, NP-9-Cz and NP-2-Cz. | ||
The zero electric field charge mobility (μ0) and field dependence parameter (α) was calculated using Poole–Frenkel relationship: μ = μ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(α√E). The TOF hole and electron mobility data for NP-3-Cz, NP-9-Cz, NP-2-Cz are summarized in Table 3. The TOF measurements revealed nearly by one order of magnitude higher hole and electron mobilities in the layer of NP-2-Cz with respect to those of the layers of NP-3-Cz and NP-9-Cz. Zero field electron mobility of the vacuum deposited layer of NP-2-Cz was found to be higher than 10−3 cm2 V−1 s−1 at room temperature. At the electric field of 2.5 × 105 V cm−1 hole-mobility values of the layers of NP-3-Cz, NP-9-Cz, NP-2-Cz were found to be 9.1 × 10−4, 2.5 × 10−4, 9.3 × 10−3 cm2 V−1 s−1, while the electron-mobility values were 5.4 × 10−5, 1.3 × 10−4, 2.6 × 10−3, 2.6 × 10−3 cm2 V−1 s−1, respectively. The highest charge mobilities in the layers NP-2-Cz can apparently be explained by more effective conjugation of electrons between perylene and carbazole moieties. This conclusion is in a good agreement with the lowest value of λh for the NP-2-Cz molecule, while the λh value contradicts to the experiment that can be explained by the crucial role of charge transfer integrals, which depend on the crystal structure of the NP-2-Cz (this aspect have not taken into account in the realized DFT calculations).
exp(α√E). The TOF hole and electron mobility data for NP-3-Cz, NP-9-Cz, NP-2-Cz are summarized in Table 3. The TOF measurements revealed nearly by one order of magnitude higher hole and electron mobilities in the layer of NP-2-Cz with respect to those of the layers of NP-3-Cz and NP-9-Cz. Zero field electron mobility of the vacuum deposited layer of NP-2-Cz was found to be higher than 10−3 cm2 V−1 s−1 at room temperature. At the electric field of 2.5 × 105 V cm−1 hole-mobility values of the layers of NP-3-Cz, NP-9-Cz, NP-2-Cz were found to be 9.1 × 10−4, 2.5 × 10−4, 9.3 × 10−3 cm2 V−1 s−1, while the electron-mobility values were 5.4 × 10−5, 1.3 × 10−4, 2.6 × 10−3, 2.6 × 10−3 cm2 V−1 s−1, respectively. The highest charge mobilities in the layers NP-2-Cz can apparently be explained by more effective conjugation of electrons between perylene and carbazole moieties. This conclusion is in a good agreement with the lowest value of λh for the NP-2-Cz molecule, while the λh value contradicts to the experiment that can be explained by the crucial role of charge transfer integrals, which depend on the crystal structure of the NP-2-Cz (this aspect have not taken into account in the realized DFT calculations).
Performance in organic light emitting diodes
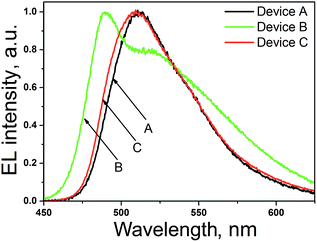
Electroluminescence spectra of the fabricated OLEDs were found to be similar to the PL spectrum of the films of NP-3-Cz, NP-9-Cz and NP-2-Cz (Fig. 9). Thus, the layers of the newly synthesized compounds were responsible for the OLED emission. The EL spectrum of device B was found to be wider with lower-energy maximum at 490 nm.The basic characteristics of the fabricated devices A, B and C are presented in Table 4. The high brightness of fabricated devices (Fig. 10) can be explain the high emission quantum yields of the films of NP-3-Cz, NP-9-Cz and NP-2-Cz and ambipolar charge-transporting properties of these compounds. The best characteristics observed for device C can apparently be explained by favourable charge-transporting properties of NP-2-Cz. Moreover, LUMO energy levels of NP-3-Cz and NP-2-Cz matched with the energy levels of the anodes, that provide low turn-on voltage of the devices (2.0 V) (Fig. 10).
| Device | Max. EQE and current efficiency | Max. power efficiency | Turn-on voltage | Max. brightness |
|---|---|---|---|---|
| A | 3.7% 12.9 cd A−1 | 8.8 lm W−1 | 2.0 V | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cd m−2 (11.8 V, 220 mA cm−2) 500 cd m−2 (11.8 V, 220 mA cm−2) |
| B | 1.9% 6.5 cd A−1 | 5 lm W−1 | 2.2 V | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cd m−2 (15 V, 425 mA cm−2) 300 cd m−2 (15 V, 425 mA cm−2) |
| C | 4.2% 14.6 cd A−1 | 11.4 lm W−1 | 2.0 V | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 (15 V, 450 mA cm−2) 000 cd m−2 (15 V, 450 mA cm−2) |
Experimental
Instrumentation
1H and 13C NMR spectra were recorded using Varian Unity Inova (300 MHz (1H), 75.4 MHz (13C)) and Bruker Avance III 400 spectrometer (400 MHz (1H), 100 MHz (13C)) apparatus. Infrared (IR) spectra were recorded on Perkin Elmer Spectrum GX spectrometer. The spectra of the solid compounds were recorded using KBr pellets. Mass (MS) spectra were obtained on Bruker maxis 4G. Elemental analysis was performed with an Exeter Analytical CE-440 Elemental. Melting points (mp) of the synthesized compounds were estimated using Electrothermal Mel-Temp apparatus. Elemental analysis was performed with an Exeter Analytical CE-440 Elemental.UV spectra were recorded with Aventes AvaSpec-2048XL spectrometer. Fluorescence (FL) spectra, fluorescence quantum yields and fluorescence decay curves of the dilute THF solutions and of the solid films of the compounds were recorded by Edinburgh Instruments FLS980 spectrometer.
Differential scanning calorimetry (DSC) measurements were carried out in a nitrogen atmosphere with a Perkin Elmer at DSC 8500 equipment at heating rate of 10 °C min−1. Thermogravimetric analysis (TGA) was performed on a Perkin Elmer TGA 4000 apparatus in a nitrogen atmosphere at a heating rate of 10 °C min−1.
Cyclic voltammetry (CV) measurements were carried out by a three-electrode assembly cell from a Bio-Logic SAS and a micro-AUTOLAB Type III potentiostat–galvanostat. The measurements were carried out with a glassy carbon electrode in dichloromethane solutions containing 0.1 M tetrabutylammonium hexafluorphosphate as electrolyte, Ag/AgNO3 as the reference electrode, and a Pt wire counter electrode.
Charge carrier mobility (μ) measurements of the vacuum deposited layers with the thickness varying from 1.0 to 4.2 nm of the compounds were carried out by the time of flight method (TOF).25 The sandwich-like samples (ITO/compounds/Al) were used for the measurements. The ionization energies (IP) of the films of the synthesized compounds were measured by an electron photoemission in air method as described before.26 The measurement method was, in principle, similar to the described in literature.27
Organic light emitting diodes (OLEDs) with the structures: ITO/CuI (8 nm)/DMAC36 (20 nm)/NP-3-Cz (30 nm)/Bphen (20 nm)/Ca (50 nm)/Al (200 nm) (device A), ITO/CuI (8 nm)/DMAC36 (20 nm)/NP-9-Cz (30 nm)/Bphen (20 nm)/Ca (50 nm)/Al (200 nm) (device B), ITO/CuI (8 nm)/DMAC36 (20 nm)/NP-2-Cz (30 nm)/Bphen (20 nm)/Ca (50 nm)/Al (200 nm) (device C) were fabricated by step-by-step deposition of different organic layers and metal electrodes onto precleaned indium-tin-oxide (ITO)-coated glass substrate under a vacuum of 10−5 Torr. CuI and 3,6-di[di(4-methylphenyl)amino]-9-ethylcarbazole (DMAC36) were used for the preparation of hole-transporting layers,9 Bphen was applied as the electron transporting layer.28 Since Ca is highly reactive and corrodes quickly in the ambient atmosphere, Ca layer topped with 200 nm aluminum (Al) layer was used as the cathode. The active area of the obtained devices was 2 × 3 mm2. The density current–voltage and luminance–voltage characteristics were measured using a semiconductor parameter analyzer (HP 4145A) in air without passivation immediately after the formation of the device. The brightness measurements were done using a calibrated photodiode.9 The electroluminescence spectra were recorded with an Ocean Optics USB2000 spectrometer.
Materials
Carbazole, N-bromosuccinimide (NBS), chloroform, potassium hydroxide, copper(I) iodide, dry methanol, dry dimethylformamide (DMF), sodium, 2-ethylhexylbromide, 1-Br-3-methylbutane, potassium carbonate, acetone, 1,2-dichlorobenzene (o-DCB), 18-crown-6, copper, tetrabutylammonium hydrogensulfate, n-butyllithium, 2-iso-propoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, bis(triphenylphosphine) palladium(II) dichloride, tetrahydrofurane (THF), 1,4-dioxane, perylene, triphenylphosphine were purchased from Aldrich and used as received without further purification.1-(2-Ethylhexyl)-1H-phenanthro[1,10,9,8-cdefg]carbazole (A-NP)
To the solution of 1H-phenanthro[1,10, 9,8-cdefg]carbazole (H-NP) (1.1 g, 4.2 mmol) in acetone (100 mL) potassium carbonate (0.23 g, 1.7 mmol), potassium hydroxide (0.70 g, 12.5 mmol), catalytic amount of tetrabutylammonium hydrogen sulfate and an excess of 2-ethylhexylbromide (0.96 g, 5.0 mmol) were added. The reaction mixture was allowed to reflux for 2 hours. After TLC control the reaction mixture was cooled down to the room temperature, treated with ethyl acetate and washed with distilled water. The organic layer was dried over anhydrous Na2SO4, filtered and the solvents were removed. After the column chromatography which was performed using acetone/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as an eluent compound A-NP (1.25 g, 80%), was obtained as yellow crystals. 1H NMR (700 MHz, CDCl3) δ 8.64 (d, J = 7.4 Hz, 2H), 8.12 (d, J = 7.9 Hz, 2H), 7.89 (d, J = 8.7 Hz, 2H), 7.80 (t, J = 7.7 Hz, 2H), 7.75 (d, J = 8.7 Hz, 2H), 4.55–4.47 (m, 2H), 2.24–2.17 (m, 1H), 1.47–1.23 (m, 8H), 0.94 (t, J = 7.5 Hz, 3H), 0.87 (t, J = 7.3 Hz, 3H). 13C NMR (176 MHz, CDCl3) δ 132.3, 130.5, 128.9, 125.1, 125.0, 124.6, 123.7, 120.8, 117.5, 113.6, 49.9, 41.51, 31.1, 28.9, 24.4, 23.1, 14.2, 10.9.
40) as an eluent compound A-NP (1.25 g, 80%), was obtained as yellow crystals. 1H NMR (700 MHz, CDCl3) δ 8.64 (d, J = 7.4 Hz, 2H), 8.12 (d, J = 7.9 Hz, 2H), 7.89 (d, J = 8.7 Hz, 2H), 7.80 (t, J = 7.7 Hz, 2H), 7.75 (d, J = 8.7 Hz, 2H), 4.55–4.47 (m, 2H), 2.24–2.17 (m, 1H), 1.47–1.23 (m, 8H), 0.94 (t, J = 7.5 Hz, 3H), 0.87 (t, J = 7.3 Hz, 3H). 13C NMR (176 MHz, CDCl3) δ 132.3, 130.5, 128.9, 125.1, 125.0, 124.6, 123.7, 120.8, 117.5, 113.6, 49.9, 41.51, 31.1, 28.9, 24.4, 23.1, 14.2, 10.9.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as an eluent to obtain compound (2-BCz) (0.74 g, 64%) as yellowish crystals. 1H NMR (700 MHz, CDCl3) δ 8.13 (t, J = 7.7 Hz, 2H), 7.89 (s, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.50 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.24 (t, J = 7.4 Hz, 1H), 4.39–4.36 (m, 2H), 1.79–1.72 (m, 3H), 1.42 (s, 12H), 1.05 (d, J = 6.3 Hz, 6H); 13C NMR (176 MHz, CDCl3) δ 140.9, 140.0, 126.3, 125.5, 125.1, 122.8, 121.0, 119.8, 118.8, 115.1, 115.0, 108.8, 83.9, 41.48, 37.8, 26.3, 25.1, 22.9.
40) as an eluent to obtain compound (2-BCz) (0.74 g, 64%) as yellowish crystals. 1H NMR (700 MHz, CDCl3) δ 8.13 (t, J = 7.7 Hz, 2H), 7.89 (s, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.50 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.24 (t, J = 7.4 Hz, 1H), 4.39–4.36 (m, 2H), 1.79–1.72 (m, 3H), 1.42 (s, 12H), 1.05 (d, J = 6.3 Hz, 6H); 13C NMR (176 MHz, CDCl3) δ 140.9, 140.0, 126.3, 125.5, 125.1, 122.8, 121.0, 119.8, 118.8, 115.1, 115.0, 108.8, 83.9, 41.48, 37.8, 26.3, 25.1, 22.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to obtain 3Br-ACz as slightly yellowish crystals (yield 83%). 1H NMR (700 MHz, CDCl3) δ 8.22 (d, J = 2.0 Hz, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.56 (dd, J = 8.6, 1.9 Hz, 1H), 7.52 (t, J = 7.1 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.29–7.25 (m, 2H), 4.25 (d, J = 15.2 Hz, 2H), 1.75–1.67 (m, 3H), 1.04 (d, J = 6.6 Hz, 6H). 13C NMR (176 MHz, CDCl3) δ 140.6, 139.0, 128.3, 126.4, 124.7, 123.2, 121.9, 120.7, 119.3, 111.6, 110.1, 108.9, 41.5, 37.5, 26.2, 22.7.
40) to obtain 3Br-ACz as slightly yellowish crystals (yield 83%). 1H NMR (700 MHz, CDCl3) δ 8.22 (d, J = 2.0 Hz, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.56 (dd, J = 8.6, 1.9 Hz, 1H), 7.52 (t, J = 7.1 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.29–7.25 (m, 2H), 4.25 (d, J = 15.2 Hz, 2H), 1.75–1.67 (m, 3H), 1.04 (d, J = 6.6 Hz, 6H). 13C NMR (176 MHz, CDCl3) δ 140.6, 139.0, 128.3, 126.4, 124.7, 123.2, 121.9, 120.7, 119.3, 111.6, 110.1, 108.9, 41.5, 37.5, 26.2, 22.7.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as an eluent to obtain compound NP-3-Cz (0.32 g, 58%) as a yellow crystals. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 7.5 Hz, 2H), 8.46 (s, 2H), 8.23 (d, J = 8.3 Hz, 2H), 8.17 (d, J = 7.7 Hz, 2H), 7.90–7.83 (m, 4H), 7.78 (t, J = 7.9 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.55–7.45 (m, 4H), 7.29–7.22 (m, 2H), 4.61 (d, J = 7.5 Hz, 2H), 4.41 (t, J = 7.9 Hz, 4H), 2.37–2.27 (m, 1H), 1.90–1.74 (m, 6H), 1.47–1.22 (m, 8H), 1.09 (d, J = 6.3 Hz, 12H), 0.94 (t, J = 7.4 Hz, 3H), 0.83 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 140.9, 139.7, 138.3, 132.9, 132.5, 130.9, 128.6, 128.4, 125.9, 125.1, 124.7, 124.6, 123.2, 123.1, 122.2, 120.9, 120.7, 119.0, 116.8, 114.7, 108.9, 108.5, 50.0, 41.7, 41.3, 37.8, 30.9, 28.7, 26.4, 24.3, 23.2, 22.8, 14.2, 11.0.
40) as an eluent to obtain compound NP-3-Cz (0.32 g, 58%) as a yellow crystals. 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 7.5 Hz, 2H), 8.46 (s, 2H), 8.23 (d, J = 8.3 Hz, 2H), 8.17 (d, J = 7.7 Hz, 2H), 7.90–7.83 (m, 4H), 7.78 (t, J = 7.9 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.55–7.45 (m, 4H), 7.29–7.22 (m, 2H), 4.61 (d, J = 7.5 Hz, 2H), 4.41 (t, J = 7.9 Hz, 4H), 2.37–2.27 (m, 1H), 1.90–1.74 (m, 6H), 1.47–1.22 (m, 8H), 1.09 (d, J = 6.3 Hz, 12H), 0.94 (t, J = 7.4 Hz, 3H), 0.83 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 140.9, 139.7, 138.3, 132.9, 132.5, 130.9, 128.6, 128.4, 125.9, 125.1, 124.7, 124.6, 123.2, 123.1, 122.2, 120.9, 120.7, 119.0, 116.8, 114.7, 108.9, 108.5, 50.0, 41.7, 41.3, 37.8, 30.9, 28.7, 26.4, 24.3, 23.2, 22.8, 14.2, 11.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as an eluent to obtain compound NP-2-Cz (0.28 g, 51%) as a yellow crystals. 1H NMR (700 MHz, CDCl3) δ 8.77–8.71 (m, 2H), 8.31–8.24 (m, 4H), 8.22 (d, J = 7.6 Hz, 2H), 7.94 (s, 2H), 7.83–7.80 (m, 2H), 7.79 (s, 2H), 7.64 (d, J = 7.7 Hz, 2H), 7.53 (t, J = 7.2 Hz, 2H), 7.48 (d, J = 8.1 Hz, 2H), 7.31 (t, J = 7.0 Hz, 2H), 4.70–4.62 (m, 2H), 4.43–4.39 (m, 4H), 2.35 (d, J = 37.6 Hz, 1H), 1.85 (dd, J = 15.1, 6.9 Hz, 4H), 1.79–1.73 (m, 2H), 1.51–1.26 (m, 8H), 1.04 (d, J = 6.6 Hz, 12H), 0.98 (t, J = 7.4 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H); 13C NMR (176 MHz, CDCl3) δ 141.0, 140.7, 139.8, 138.6, 132.5, 130.9, 128.4, 128.3, 125.8, 125.1, 124.7, 123.0, 122.2, 121.8, 121.1, 120.6, 120.4, 119.1, 117.0, 114.7, 110.5, 108.8, 77.3, 77.2, 77.0, 68.1, 50.0, 41.6, 41.4, 37.8, 31.0, 28.9, 26.3, 24.3, 23.2, 22.8, 14.2, 10.9.
40) as an eluent to obtain compound NP-2-Cz (0.28 g, 51%) as a yellow crystals. 1H NMR (700 MHz, CDCl3) δ 8.77–8.71 (m, 2H), 8.31–8.24 (m, 4H), 8.22 (d, J = 7.6 Hz, 2H), 7.94 (s, 2H), 7.83–7.80 (m, 2H), 7.79 (s, 2H), 7.64 (d, J = 7.7 Hz, 2H), 7.53 (t, J = 7.2 Hz, 2H), 7.48 (d, J = 8.1 Hz, 2H), 7.31 (t, J = 7.0 Hz, 2H), 4.70–4.62 (m, 2H), 4.43–4.39 (m, 4H), 2.35 (d, J = 37.6 Hz, 1H), 1.85 (dd, J = 15.1, 6.9 Hz, 4H), 1.79–1.73 (m, 2H), 1.51–1.26 (m, 8H), 1.04 (d, J = 6.6 Hz, 12H), 0.98 (t, J = 7.4 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H); 13C NMR (176 MHz, CDCl3) δ 141.0, 140.7, 139.8, 138.6, 132.5, 130.9, 128.4, 128.3, 125.8, 125.1, 124.7, 123.0, 122.2, 121.8, 121.1, 120.6, 120.4, 119.1, 117.0, 114.7, 110.5, 108.8, 77.3, 77.2, 77.0, 68.1, 50.0, 41.6, 41.4, 37.8, 31.0, 28.9, 26.3, 24.3, 23.2, 22.8, 14.2, 10.9.Computational details
The equilibrium structural parameters of the NP-9-Cz, NP-3-Cz, NP-2-Cz molecules were optimized at the B3LYP/6-31G(d)36–38 level of the density functional theory (DFT) using the Gaussian 09 software package.39 We have also calculated the vibrational frequencies for the studied compound in order to verify determination of the true minimum on potential energy surface (PES). All vibrational frequencies are found to be real, which indicates the location of the sought-for energy minimum. The electronic absorption spectra of the studied molecules have been calculated by the time dependent (TD) DFT method40 in the THF medium accounting the polarized continuum model (PCM)41 using the same B3LYP/6-31G(d) approach.Reorganization energy values for the electron (λ−) and hole (λ+) carriers were calculated using the following equation being widely used for estimation of the charge transport properties of organic materials:42
| λ± = (E*± − E±) + (E**± − E0), | (1) |
Conclusions
N-annelated perylenes containing two differently linked carbazolyl substituents were synthesized and their thermal, optical, photophysical and electrochemical properties were studied. The synthesized compounds were found to be efficient green-emitting fluorophores. They were used for the fabrication of the emissive layers of the effective and highly luminous OLEDs. The brightness value exceeding 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and external quantum efficiency of 4.2% were recorded. We suggest that such high efficiencies of the fabricated devices are due to the high fluorescence quantum yields of compounds in the solid state as well as due to high and balanced electron and hole motilities. The TDDFT calculated values of the S0–S1 oscillator strengths were found to be very high that causes the high fluorescence ability. The oscillator strengths (0.9, 0.49, 1.06) are in a good correlation with the brightness (14
000 cd m−2 and external quantum efficiency of 4.2% were recorded. We suggest that such high efficiencies of the fabricated devices are due to the high fluorescence quantum yields of compounds in the solid state as well as due to high and balanced electron and hole motilities. The TDDFT calculated values of the S0–S1 oscillator strengths were found to be very high that causes the high fluorescence ability. The oscillator strengths (0.9, 0.49, 1.06) are in a good correlation with the brightness (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, 31
500, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and 62
300 and 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2) and EQE (3.7, 1.9, 4.2) values observed for the fabricated OLEDs.
000 cd m−2) and EQE (3.7, 1.9, 4.2) values observed for the fabricated OLEDs.
Acknowledgements
This work was financially supported by the Taiwan-Latvia-Lithuania cooperation project "Synthesis and studies of organic electroactive materials for effective and reliable optoelectronic devices" (TAPLLT1/13). We thank Professor Hans Ågren (KTH, Stockholm) for the PDC supercomputer use. All calculations were performed with resources provided by the Swedish National Infrastructure for Computing (SNIC) at the Parallel Computer Center (PDC) through the project “Multiphysics Modeling of Molecular Materials”, SNIC 020/11-23. This research was also supported by the Ministry of Education and Science of Ukraine (project number 0115U000637). Boris F. Minaev acknowledges the grant of the Chinese Academy of Science in the framework of the President's International Fellowship Initiative for Visiting Scientists in 2015.References
- Y. Zhu, A. P. Kulkarni, P.-T. Wu and S. A. Jenekhe, Chem. Mater., 2008, 20, 4200 CrossRef CAS.
- A. L. Fisher, K. E. Linton, K. T. Kamtekar, C. Pearson, M. R. Bryce and M. C. Petty, Chem. Mater., 2011, 23, 1640 CrossRef CAS.
- J. Wu, S. Wu, Y. Geng, G. Yang, S. Muhammad, J. Jin, Y. Liao and Z. Su, Theor. Chem. Acc., 2010, 127, 419 CrossRef CAS.
- L. Duan, Q. Juan, S. Yongduo and Y. Qiu, Adv. Mater., 2011, 23, 1137 CrossRef CAS PubMed.
- D. Gudeika, A. Michaleviciute, J. V. Grazulevicius, R. Lygaitis, S. Grigalevicius, V. Jankauskas, A. Miasojedovas, S. Jursenas and G. Sini, J. Phys. Chem. C, 2012, 116, 14811 CAS.
- C. R. McNeill and N. C. Greenham, Adv. Mater., 2009, 21, 3840 CrossRef CAS PubMed.
- W.-Y. Hung, G.-C. Fang, S.-W. Lin, S.-H. Cheng, K.-T. Wong, T.-Y. Kuo and P.-T. Chou, Sci. Rep., 2014, 4, 5161 CAS.
- D.-Y. Zhou, H. Z. Siboni, Q. Wang, L.-S. Liao and H. Aziz, J. Phys. Chem. C, 2014, 118, 24006 CAS.
- D. Volyniuk, V. Cherpak, P. Stakhira, B. F. Minaev, G. V. Baryshnikov, M. Chapran, A. Tomkeviciene, J. Keruckas and J. V. Grazulevicius, J. Phys. Chem. C, 2013, 117, 22538 CAS.
- B. Minaev, G. Baryshnikov and H. Agren, Phys. Chem. Chem. Phys., 2014, 16, 1719 RSC.
- V. Cherpak, P. Stakhira, B. Minaev, G. Baryshnikov, E. Stromylo, I. Helzhynskyy, M. Chapran, D. Volyniuk, D. Tomkuté-Luksiené, T. Malinauskas, V. Getautis, A. Tomkeviciene, J. Simokaitiene and J. V. Grazulevicius, J. Phys. Chem. C, 2014, 118, 11271 CAS.
- V. Cherpak, P. Stakhira, B. Minaev, G. Baryshnikov, E. Stromylo, I. Helzhynskyy, M. Chapran, D. Volyniuk, Z. Hotra, A. Dabuliene, A. Tomkeviciene, L. Voznyak and J. V. Grazulevicius, ACS Appl. Mater. Interfaces, 2015, 7, 1219 CAS.
- R. R. Reghu, H. K. Bisoyi, J. V. Grazulevicius, P. Anjukandi, V. Gaidelis and V. J. Jankauskas, J. Mater. Chem., 2011, 21, 7811 RSC.
- F. Wurthner, Chem. Commun., 2004, 1564 RSC.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876 CrossRef CAS PubMed.
- B. A. Jones, A. Facchetti, M. R. Wasielewski and T. J. Marks, J. Am. Chem. Soc., 2007, 129, 15259 CrossRef CAS PubMed.
- K. R. J. Thomas, J. T. Lin, Y.-T. Tao and C. H. Chuen, Mater. Chem. Phys., 2002, 12, 3516 Search PubMed.
- K. Brunner, A. van Dijken, H. Börner, J. J. A. M. Bastiaansen, N. M. M. Kiggen and B. M. W. Langeveld, J. Am. Chem. Soc., 2004, 126, 6035 CrossRef CAS PubMed.
- A. Dobarro and D. Velasco, Macromol. Chem. Phys., 1997, 198, 2563 CrossRef CAS PubMed.
- H. Meng, Z. K. Chen, X.-L. Liu, Y.-H. Lai, S.-J. Chua and W. Huang, Phys. Chem. Chem. Phys., 1999, 1, 3123 RSC.
- A. Tomkeviciene, J. V. Grazulevicius, K. Kazlauskas, A. Gruodis, S. Jursenas, T.-H. Ke and C.-C. Wu, J. Phys. Chem. C, 2011, 115, 4887 CAS.
- Z. Q. Gao, M. Luo, X. H. Sun, H. L. Tam, M. S. Wong, B. X. Mi, P. F. Xia, K. W. Cheah and C. H. Chen, Adv. Mater., 2009, 21, 688 CrossRef CAS PubMed.
- B. W. D'andrade, S. Datta, S. R. Forrest, P. Djurovich, E. Polikarpov and M. E. Thompson, Org. Electron., 2005, 6, 11 CrossRef PubMed.
- J. L. Bredas, R. Silbey, D. S. Boudreaux and R. R. Chance, J. Am. Chem. Soc., 1983, 105, 6555 CrossRef CAS.
- P. M. Borsenberger and D. S. Weiss, Organic photoreceptors for xerography, Dekker, New York, 1998, p. 768 Search PubMed.
- N. A. Kukhta, D. Volyniuk, L. Peciulyte, J. Ostrauskaite, G. Juska and J. V. Grazulevicius, Dyes Pigm., 2015, 117, 122 CrossRef CAS PubMed.
- E. Miyamoto, Y. Yamaguchi and M. Yokoyama, Electrophotography, 1989, 28, 364 CAS.
- S. Naka, H. Okada, H. Onnagawa and T. Tsutsui, Appl. Phys. Lett., 2000, 76, 197 CrossRef CAS PubMed.
- J.-C. Li, Q. B. Meng, J. S. Meng, Y. S. Kim and B. A. Lee, Bull. Korean Chem. Soc., 2009, 30, 951 CrossRef CAS.
- A. W. Freeman, M. Urvoy and M. E. Criswell, J. Org. Chem., 2005, 70, 5014 CrossRef CAS PubMed.
- W. Jiang, H. Qian, Y. Li and Z. Wang, J. Org. Chem., 2008, 73, 7369 CrossRef CAS PubMed.
- J. J. Looker, J. Org. Chem., 1972, 37, 3379 CrossRef CAS.
- W. Tang, T. Lin, L. Ke and Z. Chen, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7725 CrossRef CAS PubMed.
- S. H. Tucker, J. Chem. Soc., 1926, 129, 546 RSC.
- S. Gauthier and J. M. J. Frechet, Synthesis, 1987, 4, 383 CrossRef.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, D. J. DeFrees, J. A. Pople and M. S. Gordon, J. Chem. Phys., 1982, 77, 3654 CrossRef CAS PubMed.
- Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2009 Search PubMed.
- E. Runge and E. K. U. Gross, Phys. Rev. Lett., 1984, 52, 997 CrossRef CAS.
- S. Miertus, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117 CrossRef CAS.
- A. Datta, S. Mohakud and S. K. Pati, J. Chem. Phys., 2007, 126, 144710 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |