Hybrid biomaterial based on porous silica nanoparticles and Pluronic F-127 for sustained release of sildenafil: in vivo study on prostate cancer†
Abstract
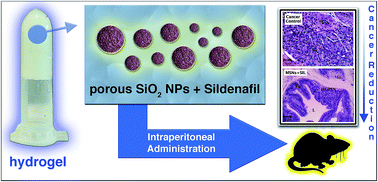
We describe here a drug depot hydrogel system comprising sildenafil (SIL; commercially available as Viagra®) incorporated in mesoporous silica nanoparticles (MSNs; around 60 nm of diameter) and conjugated with a thermosensitive poloxamer (Pluronic F-127 or PF127; possessing a liquid–gel transition over a determined temperature). The conjugation of these components results in an anticancer biomaterial with optimized physico-chemical properties: (i) MSNs are colloidally stabilized mainly through repulsive depletion forces manifested in PF127 concentrations above 5 wt%; (ii) the gelation temperature of the systems is set to about 20 °C (attained with 18 wt% of PF127) in order to result in prompt gelation when they are intraperitoneally administered (in rats) as liquids; (iii) and the hydrogel is able to induce a prolonged release of MSNs + SIL. After testing these new formulations against chemically induced prostate cancer in rats, the histopathological analyses of the prostate showed that the hybrid systems were able to largely reduce the prostate tumor level, and also highlighted the role of the MSNs in this process, since the improvement of the tumor condition was proportional to the nanoparticles concentration.

 Please wait while we load your content...
Please wait while we load your content...