Development of a multifunctional TiO2/MWCNT hybrid composite grafted on a stainless steel grating†
Abstract
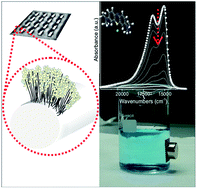
Functional materials have a promising potential for the fabrication of new devices with improved properties to meet many requirements, including environmental issues. Along this idea, a multiphase structure made using a TiO2/MWCNT hybrid nanoscaffold grafted on a metal grating (stainless steel type), acting as a strong, highly durable and heat/thermal inert support, is proposed. The method, consisting firstly of the fabrication of a porous scaffold via catalytic-CVD of a MWCNT forest on stainless steel, followed by the grafting of nanocrystalline TiO2 via the sol–gel method and then calcination, is simple and effective. Morphology, structure and optical properties have been investigated using XRD, SEM, AFM, and HRTEM techniques as well as Raman and UV-visible spectroscopy, and porosity analysis. Interestingly, the TiO2/MWCNT hybrid composite exhibits enhanced photocatalytic activity as compared to nanocrystalline TiO2, obtained by adopting the same preparation. More interestingly, the hybrid system exhibits additional functionalities, such as magnetic, surface and optical properties. The multifunctional approach allows for the combination of enhanced photodegradation with magnetic properties, which can make the recovery of a solution from a photocatalyst easier. Furthermore, it will be shown that, by moving from MWCNT/stainless steel to TiO2/MWCNT/stainless steel composites, the surface character changes from hydrophobic to hydrophilic in nature. Grafting on the stainless steel support allows for the addition of a broad range of features, including combined strength and corrosion resistance in aqueous solutions at ambient temperature, together with enhancement of electrical, optical and photocatalytic properties.

 Please wait while we load your content...
Please wait while we load your content...