Photochemically synthesized palladium nanoparticles with catalytic activity at ppb levels for C–C coupling reactions†
Abhijit Paula,
Debnath Chatterjeea,
Rajkamala,
Srirupa Banerjeeb and
Somnath Yadav*a
aDepartment of Applied Chemistry, Indian School of Mines, Dhanbad, 826004, Jharkhand, India. E-mail: yadav.s.ac@ismdhanbad.ac.in; Fax: +91-326-229-6563; Tel: +91-326-223-5880
bDepartment of Chemistry, Taki Government College, Taki, N. 24 Parganas, 743429, West Bengal, India
First published on 13th August 2015
Abstract
Palladium nanoparticles (PdNPs) of mean size 3.5 nm synthesized by a UV-mediated photochemical method have been found to be very efficient catalysts in the C–C coupling reactions such as the Suzuki–Miyaura, Mizoroki–Heck and Hiyama reactions of aryl halides, including chlorides at catalyst loadings of 83 mol ppb while affording high yields and exceptionally high turnover numbers and frequencies.
The palladium catalyzed C–C bond formation reaction is one of the most useful reactions in organic synthesis and has applications in the syntheses of many molecules ranging from pharmaceuticals to material science to optical devices.1 The search for simple and efficient Pd-catalysts that are effective at low catalyst loadings and afford high turnover numbers (TONs) is essential from many considerations including green chemistry. Towards this objective several catalysts have been developed mainly based on supported palladium nanoparticles that afford the desired low catalyst loadings, together with high turnover numbers.2–4 In fact the low catalyst loadings in such reactions have been termed “homeopathic” initially by Beletskaya5 and then others.2a,6 Recently, there have been a few reports towards the search for achieving successful C–C coupling reactions using the lowest catalytic loadings.2a,4e,4g,4j To our knowledge, the lowest catalytic loading for this class of reactions has been achieved by using a polymer supported Pd(0) nanoparticles by Yamada and co-workers where the authors reported Suzuki–Miyaura reactions using catalyst loadings of 0.28 mol ppm for aryl iodides, 40 mol ppm for the bromides and 66 mol ppm for the chlorides.4e Towards the objective of achieving high catalytic efficiencies at such low catalyst loadings, the role of the size and stabilizer of the palladium nanoparticles (PdNPs) is very important and it has been demonstrated recently that the size of the most catalytically active PdNPs is in the range of 4–5 nm.7 It was also recently shown that surfactant free Pd nanoclusters, with a large number of available free active sites, were catalytically very active affording very high TONs.8 Herein, we describe the photochemical synthesis of PdNPs of size 3.5 nm that are catalytically very active even at very low catalyst loadings of 83 ppb, affording very high turnover numbers (TONs) and turnover frequencies (TOFs) for the C–C bond forming reactions with aryl iodides, bromides and very significantly also with the very unreactive chlorides.
For the synthesis of the palladium nanoparticles, we employed the photochemical route. We chose this method since the photochemical synthesis of metal nanoparticles offers some unique opportunities for the control of their size, shape, spatial and temporal features.9 The PdNPs were synthesized by photolyzing a solution of Pd(OAc)2 (2 × 10−5 mol) and 1 (0.2 mmol) in MeOH (120 mL) under anaerobic conditions using a medium pressure mercury UV lamp. TEM characterization of the solution showed the formation of Pd-NPs with good monodispersity (Fig. 1a and b). The size distribution of the PdNPs from Fig. 1b was in between 2.5 nm to 5 nm with the mean diameter about 3.5 nm (Fig. 1c). The PdNPs were also characterized by TEM-EDX (Fig. 1d), SAED (see ESI, Fig. S1†) and XPS (see ESI, Fig. S2†). The photolyzed solution containing 2 × 10−5 mol of PdNPs was used directly for catalytic applications.
 | ||
| Fig. 1 TEM images of the PdNPs at (a) 20 nm scale and (b) 5 nm scale. (c) EDX spectra of the PdNPs. (d) Size distribution of the PdNPs from image 1b. | ||
The probable mechanism of the formation of the PdNPs is outlined in Scheme 1. The mechanism is based on the previous report of the photochemical reaction of 1 where the product 2 was formed after photolysis in the presence of Cu(I) and oxygen.10 The mechanistic scheme involves formation of the π-metal complex of Pd(II) with the photochemically excited 1, followed by cyclization to the furan ring. This is then followed by the elimination of the zero-valent Pd with the concomitant loss of methyl cation (assisted by nucleophilic attack by the acetate ion) to generate the double bond. Unlike the previous report,10 the inert conditions employed here prevented the re-oxidation of the Pd(0) species which resulted in the formation of PdNPs.
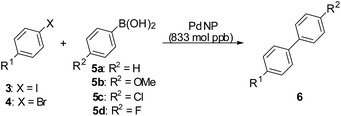
The preliminary catalytic studies with the PdNPs for the Suzuki–Miyaura11 coupling reaction were carried out with various aryl iodides and bromides (Table 1) using 5 μL of the stock solution of the PdNP catalyst [equivalent to 8.33 × 10−10 mol of Pd(0)]. For the aryl iodides, the reactions proceeded very well at a moderate temperature of 60 °C in water/methanol mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at catalyst loadings of 833 mol ppb and were completed in 3–5 hours (entries 1–11, Table 1). The reactions with the aryl bromides required a higher temperature of 100 °C (entries 12–19, Table 1). A variety of substrates with electron donating and electron withdrawing groups were explored and no significant deviations from the general reactivity was seen. The turnover numbers (TONs) achieved was of the order of 106 and the turnover frequencies (TOFs) was of the order of 105 per hour. These values are significantly higher than the conventional Pd-catalysts and quite in line with the “homeopathic” values reported for nanoparticles catalysts.2a The mercury poisoning test when carried out for the reaction between 3a and 5a, led to the complete suppression of the biphenyl formation which could be interpreted as confirmation of the participation of the PdNPs in the reactions.12
1) at catalyst loadings of 833 mol ppb and were completed in 3–5 hours (entries 1–11, Table 1). The reactions with the aryl bromides required a higher temperature of 100 °C (entries 12–19, Table 1). A variety of substrates with electron donating and electron withdrawing groups were explored and no significant deviations from the general reactivity was seen. The turnover numbers (TONs) achieved was of the order of 106 and the turnover frequencies (TOFs) was of the order of 105 per hour. These values are significantly higher than the conventional Pd-catalysts and quite in line with the “homeopathic” values reported for nanoparticles catalysts.2a The mercury poisoning test when carried out for the reaction between 3a and 5a, led to the complete suppression of the biphenyl formation which could be interpreted as confirmation of the participation of the PdNPs in the reactions.12
| Entry | 3/4 (R1) | 5 | Time, (h) | Product 6 (R1, R2) | Yieldb % | TONc [×106] | TOFd [×105] h−1 |
|---|---|---|---|---|---|---|---|
a Reaction was carried out by adding 5 μL of stock solution of PdNP catalyst to mixture of 3/4 (1.0 mmol), 5 (1.5 mmol) and K2CO3 (1 mmol) in 10 mL (i) MeOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then heating at 60 °C for aryliodides (ii) H2O and then heating at 100 °C for arylbromides under argon.b Isolated yields are reported.c Turnover number.d Turnover frequency. 1) and then heating at 60 °C for aryliodides (ii) H2O and then heating at 100 °C for arylbromides under argon.b Isolated yields are reported.c Turnover number.d Turnover frequency. |
|||||||
| 1 | 3a (H) | a | 3 | 6a (H, H) | 97 | 1.17 | 3.90 |
| 2 | 3a (H) | b | 3 | 6b (H, OMe) | 98 | 1.18 | 3.93 |
| 3 | 3a (H) | c | 3 | 6c (H, Cl) | 92 | 1.10 | 3.68 |
| 4 | 3b (Me) | a | 3 | 6d (Me, H) | 89 | 1.07 | 3.57 |
| 5 | 3b (Me) | b | 3 | 6e (Me, OMe) | 91 | 1.09 | 3.64 |
| 6 | 3b (Me) | c | 3 | 6f (Me, Cl) | 94 | 1.13 | 3.77 |
| 7 | 3c (OMe) | a | 3 | 6b (H, OMe) | 89 | 1.07 | 3.57 |
| 8 | 3c (OMe) | c | 3 | 6g (OMe, Cl) | 94 | 1.13 | 3.77 |
| 9 | 3d (NH2) | b | 3 | 6h (NH2, OMe) | 91 | 1.09 | 3.63 |
| 10 | 3e (CO2Me) | a | 5 | 6i (CO2Me, H) | 86 | 1.03 | 2.06 |
| 11 | 3e (CO2Me) | c | 3 | 6j (CO2Me, Cl) | 89 | 1.07 | 3.57 |
| 12 | 4a (Me) | a | 3 | 6d(Me, H) | 89 | 1.07 | 3.56 |
| 13 | 4a (Me) | b | 3 | 6e (Me, OMe) | 91 | 1.09 | 3.63 |
| 14 | 4a (Me) | c | 3 | 6f (Me, Cl) | 94 | 1.13 | 3.77 |
| 15 | 4b (OMe) | a | 3 | 6b (OMe, H) | 89 | 1.07 | 3.56 |
| 16 | 4b (OMe) | c | 3 | 6g (OMe, Cl) | 94 | 1.13 | 3.77 |
| 17 | 4c (COMe) | a | 7 | 6k (COMe, H) | 92 | 1.10 | 1.57 |
| 18 | 4c (COMe) | c | 5 | 6l (COMe, Cl) | 88 | 1.06 | 2.12 |
| 19 | 4c (COMe) | d | 11 | 6m (COMe, F) | 84 | 1.01 | 0.92 |
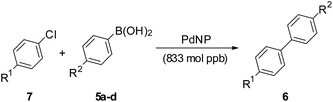
Often aryl chlorides are more easily accessible and cheaper than the corresponding iodides or bromides. However, chlorides are very less reactive and this aspect hinders their use in the Pd catalysed C–C bond formation reactions.13 In view of the high activity of the PdNP catalyst observed in the reactions with iodides and bromides, we explored the reactions of aryl chlorides (Table 2) with PdNP catalyst loadings similar to those for the iodides and bromides (833 mol ppb). A variety of substrates with substituents having different electronic effects were explored to give excellent yields of products. The TONs were again of the order of 106 and the TOFs of the order of 105.
| Entry | 7 | 5 | Time (h) | Productb [yield%] | TONc | TOFd |
|---|---|---|---|---|---|---|
| a Reaction was carried out by adding 5 μL of stock solution of PdNP catalyst to mixture of 7 (1.0 mmol), 5 (1.5 mmol) and K2CO3 (1 mmol) in 10 mL H2O and then heating at 100 °C under argon.b Yields refer to isolated yields.c Turnover number.d Turnover frequency (h−1). | ||||||
| 1 | 7a (H) | a | 6 | 6a [86] | 1.03 × 106 | 1.72 × 105 |
| 2 | 7a (H) | b | 6 | 6b [76] | 0.92 × 106 | 1.52 × 105 |
| 3 | 7a (H) | c | 6 | 6c [91] | 1.10 × 106 | 1.82 × 105 |
| 4 | 7a (H) | d | 8 | 6n [85] | 1.02 × 106 | 1.27 × 105 |
| 5 | 7b (NH2) | b | 5 | 6h [94] | 1.13 × 106 | 2.26 × 105 |
| 6 | 7c (CO2Me) | a | 12 | 6i [86] | 1.03 × 106 | 0.85 × 105 |
| 7 | 7c (CO2Me) | c | 8 | 6j [84] | 1.01 × 106 | 1.26 × 105 |
| 8 | 7c (COMe) | a | 9 | 6k [86] | 1.03 × 106 | 1.14 × 105 |
| 9 | 7c (COMe) | d | 6 | 6m [89] | 1.07 × 106 | 1.78 × 105 |
We next turned our attention towards testing the lower limits of the catalyst loadings for the Suzuki–Miyaura coupling reactions. For this, we explored reactions of the aryl halides with phenylboronic acid at 10 mmol scale of the aryl halide substrate using the same volume (5 μL) of the catalyst solution, thus effectively lowering the catalyst loadings to 83 mol ppb. The results are summarized in Table 3. The reactions were very efficient for all the substrates even at these low catalytic loadings and very good yields of the products were isolated. The TONs for the reactions were of the order of 107 except for bromide 4b and the chlorides 7d and 7e. The highest TON achieved was 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the reaction with iodobenzene (3a). For the aryl bromides, the highest TON of 10
000 for the reaction with iodobenzene (3a). For the aryl bromides, the highest TON of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved for 4a, while for the chlorides, the highest TON of 10
000 was achieved for 4a, while for the chlorides, the highest TON of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved for substrate 7a.
000 was achieved for substrate 7a.
| Entry | 3/4/7 (X, R) | Time | Productb [yield%] | TONc | TOFd (h−1) |
|---|---|---|---|---|---|
a Reaction was carried out by adding 5 μL of stock solution of PdNP catalyst to mixture of 3/4/7 (10.0 mmol), 5a (15 mmol) and K2CO3 (10 mmol) in 25 mL (i) MeOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then heating at 60 °C for aryliodides (ii) H2O and then heating at 100 °C for ArBr/ArCl, under argon.b Isolated yields.c Turnover number.d Turnover frequency. 1) and then heating at 60 °C for aryliodides (ii) H2O and then heating at 100 °C for ArBr/ArCl, under argon.b Isolated yields.c Turnover number.d Turnover frequency. |
|||||
| 1 | 3a (I, H) | 3 | 6a [98] | 1.18 × 107 | 3.92 × 106 |
| 2 | 3b (I, CH3) | 5 | 6d [84] | 1.01 × 107 | 2.02 × 106 |
| 3 | 3c (I, OMe) | 9 | 6b [85] | 1.02 × 107 | 1.14 × 106 |
| 4 | 3e (I, CO2Me) | 12 | 6i [89] | 1.07 × 107 | 8.90 × 105 |
| 5 | 4a (Br, Me) | 5 | 6d [91] | 1.09 × 107 | 2.18 × 106 |
| 6 | 4b (Br, OMe) | 12 | 6b [79] | 9.48 × 106 | 7.90 × 105 |
| 7 | 4c (Br, COMe) | 8 | 6k [88] | 1.06 × 107 | 1.32 × 106 |
| 8 | 7a (Cl, H) | 24 | 6a [84] | 1.01 × 107 | 4.21 × 105 |
| 9 | 7c (Cl, CO2Me) | 24 | 6i [79] | 9.52 × 106 | 3.97 × 105 |
| 10 | 7d (Cl, COMe) | 24 | 6k [65] | 7.81 × 106 | 3.26 × 105 |
| 11 | 7e (Cl, OMe) | 24 | 6b [71] | 8.55 × 106 | 3.56 × 105 |
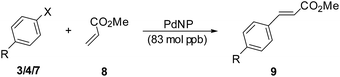
Another very important class of Pd-catalyzed C–C bond formation reaction is the Mizoroki–Heck reaction.14 Along with the Suzuki coupling reaction, the Heck reaction is one of the most explored reactions using palladium nanoparticles at very low catalyst loadings.2a,15 For the Heck reaction, the lowest catalyst loading reported is 0.9 mol ppm for ArBr6b and 490 mol ppb for ArI.4e We also explored the Heck reactions of various aryl halides including chlorides with alkenes. The reactions were carried out on a 10 mmol scale of aryl halide at catalyst loadings of 83 mol ppb. The results of the reactions with various aryl halides are presented in Table 4. In general the reactions were more efficient with the aryl iodides than the bromides and the chlorides. However, the yields even with the chlorides and the bromides were good. Intriguingly, the electron rich chloride 7c failed to react at all. The turnover numbers were of the orders of 107 for all the halides except for iodide 3d which had an unprotected NH2 substituent and the chlorides which afforded TONs of the order of 106. The highest TON of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved for the reaction with 4-methoxyiodobenzene (3c). All the aryl bromides, afforded TONs of 10
000 was achieved for the reaction with 4-methoxyiodobenzene (3c). All the aryl bromides, afforded TONs of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 while for the chlorides, the highest TON of 9
000 while for the chlorides, the highest TON of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120
120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved for substrate 7c.
000 was achieved for substrate 7c.
| Entry | 3/4/7 (X, R) | Time [h] | Productb [yield%] | TONc | TOFd |
|---|---|---|---|---|---|
| a Reaction was carried out by adding 5 μL of stock solution of PdNP catalyst to mixture of 3/4/7 (10.0 mmol), 8 (12 mmol) and Et3N (20 mmol) in 15 mL DMF and then heating at 130 °C under argon.b Isolated yields.c Turnover number.d Turnover frequency (h−1).e No reaction. | |||||
| 1 | 3a (I, H) | 5 | 9a [87] | 1.05 × 107 | 2.09 × 106 |
| 2 | 3b (I, CH3) | 4 | 9b [84] | 1.01 × 107 | 2.52 × 106 |
| 3 | 3c (I, OMe) | 8 | 9c [98] | 1.18 × 107 | 1.47 × 106 |
| 4 | 3d (I, NH2) | 5 | 9d [82] | 9.84 × 106 | 1.97 × 106 |
| 5 | 3e (I, CO2Me) | 7 | 9e [86] | 1.03 × 107 | 1.47 × 106 |
| 6 | 4a (Br, me) | 9 | 9b [84] | 1.01 × 107 | 1.12 × 106 |
| 7 | 4b (Br, OMe) | 11 | 9c [84] | 1.01 × 107 | 9.12 × 105 |
| 8 | 4c (Br, COMe) | 9 | 9f [84] | 1.01 × 107 | 1.12 × 106 |
| 9 | 7a (Cl, H) | 24 | 9a [71] | 8.52 × 106 | 3.55 × 105 |
| 10 | 7c (Cl, CO2Me) | 24 | 9e [76] | 9.12 × 106 | 3.80 × 105 |
| 11 | 7c (Cl, OMe) | 24 | NRe | — | — |
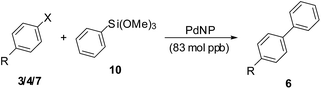
In contrast to the many reports of the Suzuki–Miyaura and the Heck coupling reactions with low catalyst loading, there are few similar studies for the Hiyama coupling reaction.15d,16 We finally attempted to study the latter reaction with the PdNP catalysts at catalyst loadings of 83 mol ppb. The reactions were carried out in water on a 10 mmol (aryl halide) scale using an excess of arylsiloxane 10 using NaOH as the base at 100 °C. The results are summarized in Table 5. The reactions with iodides required 5–9 hours for completion while those with the chloride substrates required about 24 hours for completion. Again, the yields with the aryl chlorides were slightly lesser than those for the aryl iodides or bromides. The TONs for the reactions with the aryl halides including the chlorides was of the order of 106. The TOFs for the aryl iodides and bromides were of the order of 106, while those for the aryl chlorides were of the order of 105. The highest TON achieved was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340
340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the reaction with substrate iodide 3e. Among the aryl bromides, the highest TON of 10
000 for the reaction with substrate iodide 3e. Among the aryl bromides, the highest TON of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080
080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved for 4c, while for the chlorides, a highest TON of 9
000 was achieved for 4c, while for the chlorides, a highest TON of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved for substrate 7a.
000 was achieved for substrate 7a.
| Entry | 3/4/7 (X, R) | Time [h] | Productb [yield%] | TONc | TOFd |
|---|---|---|---|---|---|
| a Reaction was carried out by adding 5 μL of stock solution of PdNP catalyst to mixture of 3/4/7 (10.0 mmol), 10 (40 mmol) and NaOH (50 mmol) in 25 mL H2O and then heating at 100 °C under argon.b Isolated yields.c Turnover number.d Turnover frequency (h−1). | |||||
| 1 | 3a (I, H) | 8 | 6a [81] | 9.72 × 106 | 1.21 × 106 |
| 2 | 3e (I, CO2Me) | 6 | 6i [86] | 10.34 × 106 | 1.72 × 106 |
| 3 | 3f (I, Me) | 5 | 6d [79] | 9.48 × 106 | 1.89 × 106 |
| 4 | 4b (Br, OMe) | 9 | 6b [68] | 8.16 × 106 | 0.91 × 106 |
| 5 | 4c (Br, COMe) | 6 | 6k [84] | 10.08 × 106 | 1.68 × 106 |
| 6 | 7a (Cl, H) | 24 | 6a [75] | 9.00 × 106 | 3.75 × 105 |
| 7 | 7c (Cl, CO2Me) | 24 | 6i [74] | 8.90 × 106 | 3.70 × 105 |
| 8 | 7d (Cl, COMe) | 24 | 6k [65] | 7.80 × 106 | 3.25 × 105 |
| 9 | 7e (Cl, OMe) | 24 | 6b [61] | 7.31 × 106 | 3.04 × 105 |
In conclusion, we have reported the efficient synthesis of Pd nanoparticles of about 3.5 nm using a photochemical method. The as synthesized PdNPs are catalytically very active for various C–C bond forming reactions of aryl halides including chlorides at very low catalytic loadings of 83 mol ppb. The TONs and the TOFs achieved at these low catalyst loadings is amongst the highest reported for the Suzuki–Miyaura and the Heck reactions and the highest for the Hiyama coupling reactions to the best of our knowledge. Further work on the applications of the PdNP catalyst for other reactions at very low catalyst loadings are in progress and will be reported in due time.
Acknowledgements
The authors thank the Science and Engineering Research Board, Department of Science and Technology, India [Grant no. SR/FT/CS-130/2011]. The NMR facilities of SAIF, Panjab University, University of Kalyani and IISER, Bhopal are gratefully acknowledged.References
- Palladium catalyzed coupling reactions, ed. Á. Molnár, Wiley-VCH Weinheim, Germany, 2013 Search PubMed.
- For reviews on the topic see: (a) C. Deraedt and D. Astruc, Acc. Chem. Res., 2014, 47, 494 CrossRef CAS PubMed and the references cited therein (b) A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973 RSC.
- K. Okumura in ref. 1 cited above.
- (a) C. Deraedt, D. Wang, L. Salmon, L. Etienne, C. Labrugere, J. Ruiz and D. Astruc, ChemCatChem, 2015, 7, 303 CrossRef CAS PubMed; (b) C. Deraedt, L. Salmon and D. Astruc, Adv. Synth. Catal., 2014, 356, 2525 CrossRef CAS PubMed; (c) V. Selivanova, V. S. Tyurin and I. P. Beletskaya, ChemPlusChem, 2014, 79, 1278 CrossRef PubMed; (d) Z. Dong and Z. Ye, Adv. Synth. Catal., 2014, 356, 3401 CrossRef CAS PubMed; (e) Y. M. A. Yamada, Y. Yuyama, T. Sato, S. Fujikawa and Y. Uozumi, Angew. Chem., Int. Ed., 2014, 53, 127 CrossRef CAS PubMed; (f) Q. M. Kainz, R. Linhardt, R. N. Grass, G. Vilé, J. Pérez-Ramirez, W. J. Stark and O. Reiser, Adv. Funct. Mater., 2014, 24, 2020 CrossRef CAS PubMed; (g) C. Deraedt, L. Salmon, L. Etienne, J. Ruiz and D. Astruc, Chem. Commun., 2013, 49, 8169 RSC; (h) M. Filice, M. Marciello, M. del Puerto Morales and J. M. Palomo, Chem. Commun., 2013, 49, 6876 RSC; (i) F. Wang, C. Li, L.-D. Sun, C.-H. Xu, J. Wang, J. C. Yu and C.-H. Yan, Angew. Chem., Int. Ed, 2012, 51, 4872 CrossRef CAS PubMed; (j) Y. M. A. Yamada, S. M. Sarkar and Y. Uozomi, J. Am. Chem. Soc., 2012, 134, 3190 CrossRef CAS PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed.
- (a) A. K. Diallo, C. Ornelas, L. Salmon, J. R. Aranzaes and D. Astruc, Angew. Chem., Int. Ed, 2007, 46, 8644 CrossRef CAS PubMed; (b) M. T. Reetz and J. G. de Vries, Chem. Commun., 2004, 1559 RSC.
- (a) M. Pérez-Lorenzo, J. Phys. Chem. Lett., 2012, 3, 167 CrossRef; (b) Y. Li and M. A. El-Sayed, J. Phys. Chem. B, 2001, 105, 8938 CrossRef CAS.
- M. Hyotanishi, Y. Isomura, H. Yamamoto, H. Kawasaki and Y. Obora, Chem. Commun., 2011, 47, 5750 RSC.
- (a) K. G. Stamplecoskie and J. C. Scaiano, J. Am. Chem. Soc., 2010, 132, 1825 CrossRef CAS PubMed; (b) J. C. Scaiano, C. Aliaga, S. Maguire and D. Wang, J. Phys. Chem. B, 2006, 110, 12856 CrossRef CAS PubMed.
- S. Kar and S. Lahiri, J. Chem. Soc., Chem. Commun., 1995, 957 RSC.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- A. S. Sigeev, A. S. Peregudov, A. V. Cheprakov and I. P. Beletskaya, Adv. Synth. Catal., 2015, 357, 417 CrossRef CAS PubMed.
- R. B. Bedford, C. S. J. Cazin and D. Holder, Coord. Chem. Rev., 2004, 248, 2283 CrossRef CAS PubMed.
- (a) T. Mizoroki, K. Mori and A. Ozaki, Bull. Chem. Soc. Jpn., 1971, 44, 581 CrossRef CAS; (b) R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320 CrossRef CAS.
- For some recent examples of Pd nanoparticle catalysed Mizoroki–Heck reactions with low catalyst loadings: (a) H. Shen, C. Shen, C. Chen, A. Wang and P. Zhang, Catal. Sci. Technol., 2015, 5, 2065 RSC; (b) D. Wong, C. Deraedt, L. Salmon, C. Labrugère, L. Etienne, J. Ruiz and D. Astruc, Chem.–Eur. J., 2015, 21, 1508 CrossRef PubMed; (c) R. L. Oliveira, J. B. F. Hooijmans, P. E. de Jongh, R. J. M. Klein Gebbink and K. P. de Jong, ChemCatChem, 2014, 6, 3223 CrossRef CAS PubMed; (d) M. Planellas, Y. Moglie, F. Alonso, M. Yus, R. Pleixats and A. Shafir, Eur. J. Org. Chem., 2014, 3001 CrossRef CAS PubMed; (e) P. Puthiaraj and K. Pitchumani, Green Chem., 2014, 16, 4223 RSC; (f) P. M. Uberman, L. A. Perez, S. E. Martin and G. I. Lacconi, RSC Adv., 2014, 4, 12330 RSC; (g) B. M. Samarasimhareddy, G. Prabhu and V. V. Sureshbabu, Tetrahedron Lett., 2014, 55, 2256 CrossRef PubMed; (h) J.-H. Noh and R. Meijboom, J. Colloid Interface Sci., 2014, 415, 57 CrossRef CAS PubMed; (i) A. L. Isfahani, I. Mohammadpoor-Baltork, V. Mirkhani, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and R. Kia, Adv. Synth. Catal., 2013, 355, 957 CrossRef CAS PubMed.
- For examples of Pd nanoparticle catalysed Hiyama coupling reactions: (a) S.-H. Huang, C.-H. Liu and C.-M. Yang, Green Chem., 2014, 16, 2706 RSC; (b) C. Premi and N. Jain, Eur. J. Org. Chem., 2013, 5493 CrossRef CAS PubMed; (c) F. Kong, C. Zhou, J. Wang, Z. Yu and R. Wang, ChemPlusChem, 2013, 78, 536 CrossRef CAS PubMed; (d) A. Grirrane, H. Garcia and A. Corma, J. Catal., 2013, 302, 49 CrossRef CAS PubMed; (e) D. Shah and H. Kaur, J. Mol. Catal. A: Chem., 2012, 359, 69 CrossRef CAS PubMed; (f) D. Srimani, A. Bej and A. Sarkar, J. Org. Chem., 2010, 75, 4296 CrossRef CAS PubMed; (g) B. C. Ranu, R. Dey and K. Chattopadhyay, Tetrahedron Lett., 2008, 49, 3430 CrossRef CAS PubMed; (h) D. Srimani, S. Sawoo and A. Sarkar, Org. Lett., 2007, 9, 3639 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, figures for XPS and SAED (TEM), compound characterization data and copies of NMR spectra available. See DOI: 10.1039/c5ra14995a |
| This journal is © The Royal Society of Chemistry 2015 |