Transfer hydrogenation of nitroarenes into anilines by palladium nanoparticles via dehydrogenation of dimethylamine borane complex†
Abstract
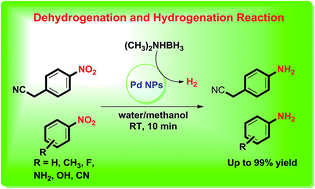
This work reports a simple and highly efficient protocol for reduction of nitroarenes to corresponding amines via dehydrogenation of dimethylamine borane using palladium nanoparticle (Pd NPs) as a versatile heterogeneous catalyst. The facile approach for the synthesis of Pd NPs within 15 min in aqueous medium has been reported. The Pd NPs were well characterized using various analytical techniques such as XRD, FEG-SEM, TEM, EDAX and XPS. The developed catalytic system uses environmentally benign dimethylamine borane as a reducing agent which is highly stable, water soluble and nontoxic. The various amines were synthesized from nitroarenes in excellent yields within 10–60 min at room temperature. The catalyst was reused up to four successive cycles without significant loss in its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...