Effect of grafted amine groups on in-plane tensile properties and high temperature structural stability of borophene nanoribbons
Jianhui Yuanab,
L. W. Zhangc and
K. M. Liew*bd
aSchool of Physics and Electronic Science, Changsha University of Science and Technology, Changsha 410114, China
bCity University of Hong Kong Shenzhen Research Institute Building, Shenzhen Hi-Tech Industrial Park, Nanshan District, Shenzhen, China. E-mail: kmliew@cityu.edu.hk; Tel: +852 3442 7601
cCollege of Information Science and Technology, Shanghai Ocean University, Shanghai 201306, China
dDepartment of Architecture and Civil Engineering, City University of Hong Kong, Kowloon, Hong Kong SAR, China
First published on 24th August 2015
Abstract
The effects of grafted amine groups on in-plane tensile properties and structural stability of armchair and zigzag borophene nanoribbons (ABNRs and ZBNRs) are investigated by using molecular dynamics. The results show that the Young’s moduli for the ABNR and ZBNR are 1.093 TPa and 0.978 TPa, respectively. Their ultimate elastic strains are respectively about 15.30% and 22.03%, showing distinct ductile and brittle fractures. When the BNRs are grafted with amine groups (–NH2), the moduli of the ABNR and ZBNR increase to 1.125 TPa and 1.016 Tpa, respectively. The ultimate elastic strain for the ABNR increases to 18.23% but that for the ZBNR gets slightly reduced to 21.12%. The fracture modes still remain unchanged. The structural deformation after being subjected to a high temperature of 1500 K shows that there is little difference between the ABNRs and ZBNRs, but the structural deformation for the grafted BNRs is obviously less than that for the non grafted BNRs. The results indicate that grafting amine groups can increase the Young’s moduli, enhance the elastic strain range, reduce the in-plane elastic anisotropy and strengthen the crack resistance. In particular, the grafting of amine groups can significantly strengthen their capacity to resist deformation at high temperature, reduce the thermal expansion anisotropy and improve the structural stability.
1. Introduction
Two-dimensional (2D) nanosheets with atomic or molecular thickness and infinite planar lengths have attracted significant interest because of their electronic, electrical and mechanical properties.1–3 Among them, as a 2D sheet of sp2 conjugated atomic carbon, graphene has attracted great attention due to its specific structure and properties such as a large surface area, good optical transparency, high thermal conductivity and mechanical strength.4,5 Graphene nanoribbons can be fabricated by lithography and can render new functionalities while preserving some of the unique properties of the pristine graphene. However, carbon’s elemental near neighbour, boron, one step to the left in the periodic table, may muscle in on graphene’s domain as the possibility of “borophene”, the boron analogue of the all-carbon material, emerges from supercomputer simulations and experimental hints.2,6Over the past decade, elemental boron clusters7–10 and the relevant low-dimensional nanostructures (fullerenes, monolayer-sheets and nanotubes, etc.)11,12 have been intriguing researchers working on molecular and/or nanoscale systems of interest as the elucidation of structures and bonding is the central focus. In particular, recent combined experimental and theoretical studies have led to a systematic understanding of the structural and bonding properties of small boron clusters suggesting a flat land of boron that is in stark contrast to bulk boron and boron alloys, in which three-dimensional (3D) structural units dominate.8,9 2D atom-thin boron sheets have attracted increasing attention.3 Early theoretical investigations have shown that graphene-like boron sheets with a honeycomb lattice are unstable; instead, boron tends to form buckled all triangular lattices.13,14 More recent theoretical studies have predicted a new type of planar boron sheets, consisting of triangular lattices with hexagonal vacancies, which are more stable and suitable to form boron nanotubes.15,16 The role of the hexagonal hole has been rationalized in terms of chemical bonding.17 Various forms of monolayer boron structures have been considered with different vacancy densities and arrangements.18–20 Piazza et al.2 provided the first experimental evidence of the viability of the novel boron nanostructures with hexagonal vacancies. These structures can indeed exist and may be synthesized using appropriate substrates.21 The potential large-scale synthesis of the new atom-thick boron nanosheets calls for an appropriate name: ‘borophene’, in analogy with graphene. They show that the structure of B36 is not only possible but highly stable.2 It is a one-atom thick disc with a perfectly symmetrical structure and the boron atoms are arranged in a triangular lattice with a perfect hexagonal hole in the middle. Photoelectron spectroscopy revealed a relatively simple spectrum, suggesting a symmetric cluster. Neutral B36 is the smallest boron cluster to have six fold symmetry and a perfect hexagonal vacancy that can be viewed as a potential basis for extended two-dimensional boron sheets. The electronic structure of the 2D boron sheets can be either metallic or semiconducting, according to theoretical calculations.18,22–24 Owing to the hexagonal holes, various chemical modifications can be made to tune the electronic and chemical properties of borophenes.25,26 Thus, borophene may constitute a new class of atom-thick nanostructures complementary to graphene. Borophene seems to have great potential for new applications in the near future due to an abundant boron resource, light atomic weight, low mass, better economy, super strong B–B atomic bonds and a special electronic structure.
In order to better understand the differences between borophene nanoribbons (BNRs) and their functional modifications in terms of structural stability and mechanical robustness, it is necessary to compare their basic elastic properties and structural states under a high-temperature and their deforming behavior under different degrees of strain. Recently, chemical functionalization of low-dimensional carbon network systems, such as carbon nanotubes (CNTs), graphene and GNRs, by introducing molecules and groups, has attracted a lot of attention; it is being viewed as a significant way to modify their physical and chemical properties.27–29 Some representative research on the effects of grafted chemical functional groups on the elastic properties of CNTs, boron nitride nanotubes and graphene, and their in-plane compressive properties and thermal stability at high temperatures, etc., has been reported.30–35 Based on these works, we further investigate the effects of grafted amine groups on armchair and zigzag BNRs (ABNRs and ZBNRs) using a molecular dynamics method. The research reported in this paper mainly calculated the Young’s moduli of ABNRs and ZBNRs with amine grafts and no grafts, optimized the geometric structures under different in-plane tensile strains and simulated the structural performance on being subjected to high temperature of 1500 K. By comparing and analyzing their moduli, critical strains and structural deformation under different conditions, etc., some new results of practical value were discovered.
2. Calculation model and method
As shown in Fig. 1, borophene, constructed from the planar hexagonal B36 unit, is an extended one-atom-thick boron sheet. The B36 unit has a perfectly symmetrical structure and its boron atoms are arranged in a triangular lattice with a perfect hexagonal hole in the middle. The circles in Fig. 1b represent the apex atoms in the B36 unit, shared by three units.2 It is one of the building blocks for boron materials in other dimensions. The BNRs (nanoribbons formed by borophene structure) can be divided into armchair and zigzag BNRs (ABNRs and ZBNRs), similar to graphene, according to the shape of the free boundary. In the simulations, the ABNR and ZBNR are set up to be about the same as the model size, i.e. 4.028 nm × 1.931 nm and 3.828 nm × 2.207 nm, respectively. The geometric structures are shown in Fig. 2. Because dangling bonds exist at atoms around the borders of the BNRs, considered to be more active, the functional groups grafted at the borders of the BNRs are often selected to enhance composite materials. Nitrogen and boron are easy to bond. Therefore, amine (–NH2) functional groups grafted in the simulations are considered here and they are linked symmetrically at the borders of the BNRs; the calculated model is shown in Fig. 3 where the functional group atom marked with an arrow is grafted to a boron atom at the borders of the BNRs. Finally, energy on the structures is minimised by geometry optimization. The conjugate gradient algorithm is applied to geometry optimization and the convergence thresholds for the specified maximum energy change, force, stress and displacement are set to 8.36 × 10−2 J mol−1, 4.18 J mol−1 Å−1, 10−3 GPa and 10−5 Å, respectively. The maximum iterations of geometry optimization cycles are 104.According to the two types of models (Fig. 2), the procedure of the simulation is as follows. To be in line with similar previous studies,30,32 in simulations, the two ends of the BNR along the x direction were clamped to apply the uniform strain along the x-direction and structural optimization. Because the clamped structure is not available for calculation and analysis of the system energies, a periodic boundary condition is applied in the x-direction after optimization. A free boundary is always set in the y-direction of the BNR. The structures with different strain conditions are optimized and the performance parameters of the optimized structures are calculated and analyzed using the universal force field (UFF).36
The UFF, a molecular mechanics force field, is where the force field parameters are estimated, using general rules based only on the element, hybridization and connectivity. The potential energy of the UFF is expressed as the sum of bonded and non-bonded interactions:
| ET = EB + EA + Eτ + Eω + Ev + Eel | (1) |
Bonded interactions include the bond stretching EB, the angle bending EA, the dihedral angle torsion Eτ and the inversion terms Eω, whereas non-bonded interactions include the van der Waals interaction Ev and the electrostatic interaction Eel. The UFF includes a parameter generator that calculates the force field parameters by combining the atomic parameters. Thus, force field parameters for any combination of different types of force fields can be generated, as required.37–39
The system strain energy is calculated using the UFF potential and its Young’s modulus is obtained by applying the classical elasticity theory
 | (2) |
To ensure thermal stability, each structure was first subjected to 1000 steps (1 ps) of relaxation, followed by 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps (10 ps) of stabilization at high temperature (1500 K) and thermal quenching at a rate of 300 K ps−1, and finally 20
000 steps (10 ps) of stabilization at high temperature (1500 K) and thermal quenching at a rate of 300 K ps−1, and finally 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps (20 ps) of equilibrium after quenching to room temperature (300 K), to obtain a stable equilibrium structure. Simulations of BNRs under tensile strain can induce geometric optimization that enables atoms in the BNRs to rotate and move in relation to one another, following a certain minimization of the strain energy in a way that allows an equilibrium state to occur. Geometric structures with different strains are collected at each tensile deformation with an incremental displacement step of 0.2 Å.
000 steps (20 ps) of equilibrium after quenching to room temperature (300 K), to obtain a stable equilibrium structure. Simulations of BNRs under tensile strain can induce geometric optimization that enables atoms in the BNRs to rotate and move in relation to one another, following a certain minimization of the strain energy in a way that allows an equilibrium state to occur. Geometric structures with different strains are collected at each tensile deformation with an incremental displacement step of 0.2 Å.
3. Results and discussion
3.1. In-plane tensile properties of BNRs
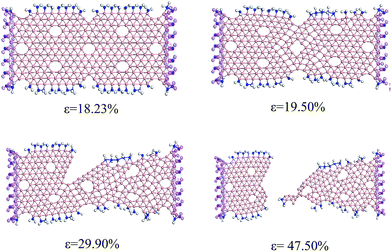
Based on our computational scheme, we optimize the geometric structure of finite BNRs and BNRs-NH2 under different in-plane tensile strains (ε), as shown in Fig. 4 and 5.Fig. 4 and 5 show the geometric structure changing process of ABNRs and ZBNRs. The results show that when the in-plane tensile strain reaches 7.7%, 15.3% and 16.7%, the ABNR begins to undergo cutting off, sleeking of a bond at the center of the border and internal hexagonal vacancies distortion but no distortion occurs in ZBNRs until the tensile strain reaches 22.0%. In particular, when the strain reaches 26.9% and 24.1%, cracks occur in the ABNR and ZBNR, respectively. The fracture modes are completely different, which shows distinct ductility for ABNRs and brittleness for ZBNRs. The crack for the ABNR begins from the middle and that for the ZBNR begins from both ends. The deformations of the ABNR have obvious elastic and plastic phases. The breakup for the ZBNR occurs so fast that one can hardly see the transitional processes. These findings suggest that there is planar stress anisotropy in the borophene.
To deeply understand the relationship between differences in moduli of the aforementioned BNRs and their structures, we further calculated the systemic energies. Fig. 6 shows the effect of different in-plane tensile strains (ε) on the total potential energy (ET) of ZBNRs and ABNRs. It’s easy to see that the ABNR and ZBNR have distinctly different elastic strains and slight differences in elastic modules. By fitting the curve around the equilibrium position, their elastic coefficients can be calculated. It’s worth pointing out that calculating the elastic modulus is meaningful only when using the thickness of the continuum assumption since the BNR membranes are composed of monolayers of boron atoms. The effective thickness of the BNR is taken as 0.34 nm, which is close to the distance between graphene layers in a graphite crystal.40–42 Through data fitting, the functional correlation between the total potential energy and the strain around the equilibrium position can be obtained as follows:
| ET = 417.48365 − 0.07544ε + 869.8ε2 for ABNRs | (3) |
| ET = 417.48529 − 0.40031ε + 845.25665ε2 for ZBNRs | (4) |
Based on formula (2), combined with formulae (3) and (4), the Young’s moduli of ABNRs and ZBNRs can be computed. The results indicate that the Young’s moduli of the BNRs are generally very large, with values for ABNRs and ZBNRs being 1.09273 TPa and 0.97804 TPa, respectively. The Young’s moduli are very close to (1.2 ± 0.1) for zigzag and (1.0 ± 0.1) TPa for armchair graphene.40,43,44 The moduli for armchairs are always larger than for zigzags, while the modulus ratio of armchair to zigzag (Ya/Yz) is 1.117, showing that there is anisotropy of in-plane elastic property of BNRs.
To further understand the essence of deformation in different phases of in-plane tensile strain, we have analyzed the bond (EB) and angle energy (EA) (see Fig. 7). By comparing Fig. 6 and 7, and combining with Fig. 4 and 5, it isn’t difficult to find that deformation features of ABNRs and ZBNRs are entirely different. The deformation process for the ABNR can be divided into four typical stages: the linear elasticity, elastic-plastic, plastic and fracture phases, but the deformation process for the ZBNR is simpler, almost straight from the linear elasticity to fracture. Fig. 6 and 7 show that the changes in the bond stretching and angle bending are the main factors that influence deformation, specially the former, among which the dominating factors are structural deformation of stretched BNRs. Fig. 6 shows the critical elastic strains of ABNRs and ZBNRs are 15.30% and 22.03%, respectively. Obviously, the critical elastic strains of ZBNRs are significantly greater than ABNRs.
 | ||
| Fig. 7 Effect of different in-plane tensile strains (ε) on the bond (EB) and angle energy (EA) of the ABNR (a) and ZBNR (b). | ||
In addition, comparing Fig. 6 and 7, one can find that the total energy ET is mainly contributed by EB and EA. Also, EA is much larger than EB, which indicates that EA dominates the ET. The main reasons are as follows: in eqn (1), the bond stretching  and the angle bending
and the angle bending  , where r − r0 and θ − θ0 are deviations of bond length or angle from equilibrium. Due to the special atomic configuration of borophene, most bond directions are not entirely tensile with direction, most of the bond length deviations r − r0 are almost very small, only when applying the strains on borophene are they not too great. In particular, almost all of the bond lengths maintain equilibrium length without the strain, their deviations r − r0 are close to zero. However, θ − θ0 is different, because the all bond angles θ in borophene under an unideal situation are more difficult to maintain their consistency, especially the many hexagonal hole defects in borophene. Consequently, EA is much larger than EB. In the expression of ET, the contribution of the latter terms is generally little and the ET is mainly contributed by the first two terms EB and EA under in-plane tensile strains in this paper.
, where r − r0 and θ − θ0 are deviations of bond length or angle from equilibrium. Due to the special atomic configuration of borophene, most bond directions are not entirely tensile with direction, most of the bond length deviations r − r0 are almost very small, only when applying the strains on borophene are they not too great. In particular, almost all of the bond lengths maintain equilibrium length without the strain, their deviations r − r0 are close to zero. However, θ − θ0 is different, because the all bond angles θ in borophene under an unideal situation are more difficult to maintain their consistency, especially the many hexagonal hole defects in borophene. Consequently, EA is much larger than EB. In the expression of ET, the contribution of the latter terms is generally little and the ET is mainly contributed by the first two terms EB and EA under in-plane tensile strains in this paper.
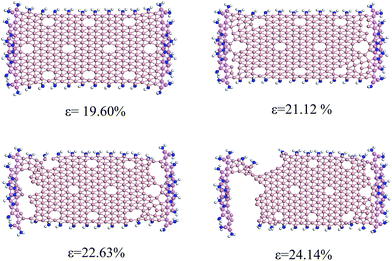
When grafted around nanoribbons with amine groups, some changes occur in all of the above structural properties of BNR-NH2 under different in-plane tensile strains. Fig. 8 and 9 show the geometric structure changing process of grafted ABNRs and ZBNRs. The results show that the bond-breaking strain in internal hexagonal vacancies at the center increase from 16.7% to 19.5% for the ABNR, while the strain for no distortion in the ZBNR decreases slightly from 22.0% to 21.1%. In particular, when the strain occurs on the BNRs, cracks obviously increase from 26.9% to 29.9% for the ABNR and from 24.1% to 22.6% for the ZBNR. The structural fracture modes for grafted BNRs still remain similar to the aforementioned non-grafted BNRs.
The effects of different in-plane tensile strains (ε) on the total potential energy (ET) of the grafted ABNR and ZBNR are as shown in Fig. 10. By comparing Fig. 6 and 10, it’s easy to see that the grafted ABNRs and ZBNRs still have distinctly different elastic strain ranges and slightly different elastic modules, as is the case with non-grafted BNRs. By fitting the curve around the equilibrium position, their elastic coefficients can be calculated. Through further data fitting, the functional correlation between the total potential energy and the strain around the equilibrium position can be obtained as follows:
| ET = 416.17487 − 0.01245ε + 895.45165ε2 for ABNR-NH2 | (5) |
| ET = 416.22776 − 0.83926ε + 878.22102ε2 for ZBNR-NH2 | (6) |
 | ||
| Fig. 10 Effect of different in-plane tensile strains (ε) on the total potential energy (ET) of BNR-NH2. | ||
Based on formula (2), combined with formulae (5) and (6), the Young’s moduli for grafted ABNRs and ZBNRs have been computed. The results indicate that the Young’s moduli for ABNR-NH2 and ZBNR-NH2 are 1.125 TPa and 1.016 TPa, respectively. The moduli for armchairs are still larger than for zigzags, and both are greater than the non-grafted BNRs. Besides, the modulus ratio of armchair to zigzag (Ya/Yz) reduces slightly from 1.117 to 1.107, showing that the anisotropy of in-plane elastic property of BNRs improves somewhat with grafted amine groups. In particular, with grafting, the critical elastic strains of ABNR-NH2 and ZBNR-NH2 are increased to 18.2% and 21.12%. Similar to the aforementioned non-grafted BNRs, the former are still greater than the latter.
Fig. 11 further shows the bond (EB) and angle energy (EA) of the ABNR-NH2 and ZBNR-NH2 with different in-plane tensile strains. Compared with non-grafted BNRs, it can be found that the strains at which EB suddenly falls significantly increases for the ABNR (from 25.65% to 36.45%) and decreases for the ZBNR (from 23.51% to 21.12%). This is consistent with the above-mentioned structural evolution and the ET change. The results indicate that the main reason for the obvious crack in the BNR is bond tolerance; grafting amine groups is useful for strengthening the crack resistance of armchair but not zigzag configurations.
 | ||
| Fig. 11 Effect of different in-plane tensile strain (ε) on the bond (EB) and angle energy (EA) of the arm (a) and zig BNR-NH2 (b). | ||
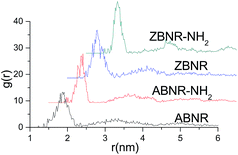
To better understand the relationship between the differences in elasticity of the aforementioned BNRs and their structures, we further investigated their radial distribution functions (RDFs). The g(r) in RDFs gives the probability of finding a B atom in the distance r from another reference B atom. If we count the appearance of two B atoms at separation r, from r = 0 to r = ∞, we can get the radial distribution function g(r). To quantify the crystalline perfection of BNRs under the different circumstances, we have computed the distribution of the inter-atomic distances from the atomic coordinates (g(r)) by using molecular dynamics. The RDF is a useful tool for describing the structure of a system. In a solid, it exhibits a number of sharp peaks, separations and heights characteristic of a lattice structure. In RDFs of BNR nanosheets, peak locations (abscissa values) correspond to the distance between the first, second and other neighboring atoms separated from the reference atom on the sheet. The ordinate values for the peaks represent that it is a relative probability that two B atoms would be found at this separation. To calculate the pair distribution function from a simulation, the neighbors around each atom or molecule are sorted into distance bins. The number of neighbors in each bin is averaged over the entire simulation. For example, a count is made of the number of neighbors for every atom or molecule in the simulation. This count can be performed during the simulation itself or by analyzing the configurations that are generated, so based on the analysis of the optimized structures, the RDF can be directly obtained from a molecular dynamics simulation. The FWHM can be obtained by calculating the abscissa difference values at the half maximum ordinate values for peaks in the RDF. In general, the full width at half maximum (FWHM) of an RDF is closely related to the integrity of the crystal structure. The more integrated the structure is, the sharper the RDF peaks are and the smaller the FWHM is, and vice versa.
Fig. 12 (Table 1) shows that there is some difference between RDF parameters in grafted and non-grafted BNRs. Locations of their highest peaks, subpeaks and third peaks are about 0.186, 0.323 and 0.371 nm, respectively, with little significant change taking place among the BNRs. But their FWHMs are different. The first three FWHMs for the ABNRs are about 0.0802, 0.1410 and 0.1235 nm, respectively. After being grafted, the FWHMs are 0.0750, 0.0907 and 0.1162 nm. It can be seen that the FWHMs for the grafted BNRs are slightly smaller than those for the non-grafted BNRs. Similarly, the FWHMs of the first three peaks for the ZBNRs are about 0.0773, 0.1450 and 0.1080 nm, respectively. After grafting, the FWHMs are 0.0760, 0.1380 and 0.1078 nm. The FWHMs for the grafted BNRs are still slightly smaller than those for the non-grafted BNRs. These results can be attributed to the differences in free boundary shapes and grafts and lead to the differences between the B–B bond lengths of the BNRs in the triangular lattices. These results indicate that grafts can decrease the FWHMs of the crystal structure, thereby improving the integrity of the crystal structure. Comparison of the FWHMs in terms of the elastic moduli, the critical elastic strains and the rupture strains before and after grafting show that the elastic properties of the BNRs are basically related to the integrity of the crystal structure. The smaller the FWHMs are, the more integrated the structure is and the stronger the distortion resistance is.
| Material | First peak | Second peak | Third peak | |||
|---|---|---|---|---|---|---|
| Location | FWHM | Location | FWHM | Location | FWHM | |
| ABNR | 0.186 | 0.0802 | 0.323 | 0.1410 | 0.371 | 0.1235 |
| ABNR-NH2 | 0.186 | 0.0750 | 0.323 | 0.0907 | 0.371 | 0.1162 |
| ZBNR | 0.186 | 0.0773 | 0.323 | 0.1450 | 0.371 | 0.1080 |
| ZBNR-NH2 | 0.186 | 0.0760 | 0.323 | 0.1380 | 0.371 | 0.1078 |
3.2. High-temperature structural stability of BNRs
As with graphene, the BNR and its composite structures are likely to be used in varying high-temperature environments in actual applications, such as the spark plasma sintering of graphene reinforced zirconium diboride ultra-high temperature ceramic composites,45 high-temperature multifunctional magneto active nickel graphene polyamide nanocomposites,46 and so on. In order to know the high-temperature structural stability of BNRs, structural performances of the above-mentioned four BNRs, when subjected to high temperatures, are simulated and analysed through molecular dynamics simulations. Fig. 13 shows the overall appearance of the four BNRs after quenching at a temperature of 1500 K. Because the difference at a lower quenching temperature is not large enough to clearly describe, a higher quenching temperature (1500 K) is selected in this paper. It is evident that the structural deformation in Fig. 13a1 and a2 is obviously greater than that in Fig. 13b1 and b2. In the former, some areas of the structures exhibit some chaos in bond orders and the hexagonal holes in the structures are obviously distorted or ambiguous, whereas those in the latter display only slight chaos in bond orders and no obvious distortions, especially near the clear hexagonal holes in the structures. At the same time, comparison of Fig. 6a1 and a2 shows that deformation of the zigzag BNR is slightly less than that of the armchair BNR but Fig. 6b1 and b2 show no evident difference between the armchair and the zigzag in terms of structural deformation. This indicates that the degree of structure distortion is related to the chirality, i.e. there is some thermal expansion anisotropy in BNRs. Therefore, grafted amine groups can not only enhance resistance to deformation at high temperature but also reduce its thermal expansion anisotropy. The geometric models of BNRs at high temperature suggest that the grafted amine groups have certain protective effects that improve the high temperature structural stability of BNRs. | ||
| Fig. 13 Geometric models of ABNR and ZBNR (a1 and a2) and ABNR-NH2 and ZBNR-NH2 (b1 and b2) after quenching at 1500 K. | ||
Fig. 14 (Table 2) shows the RDF parameters at the quenching temperature of 1500 K. The thermal expansion of atomic distances and the increase in lattice vibrational frequency occur with the rise in temperature on the basis of thermodynamics, so it is reasonable that the RDF values of the grafted and non-grafted BNRs after quenching at 1500 K (Fig. 14) have more noise than data presented in Fig. 12. From Fig. 14 (Table 2), the FWHMs of the highest peaks for the ABNR and ZBNR are 0.03558 and 0.03094 nm, respectively. When grafted, the distribution of the peak narrows. The FWHM of the highest peak for the ABNR decreases from 0.03558 to 0.02165 nm and for the ZBNR decreases from 0.03094 to 0.02011 nm. These results indicate that at a high temperature, the structure displays a smaller deviation in distance. The neighbouring atomic distances are more concentrated at all levels in the triangular lattices on the nanosheets. Hence, the crystal structures of the BNRs are protected by the amine grafts to a certain extent, suggesting that BNR-NH2 has a higher resistance to distortion at high temperature. Therefore, grafting amine groups can improve the structural stability of BNRs at high temperature. In addition, it’s easy to find that the FWHM of the ABNR is greater than ZBNR but that of the grafted ABNR is closer to that of the grafted ZBNR. This result indicates that grafting amine groups effectively reduces the thermal expansion anisotropy of BNRs. The RDF quantitative analysis result is consistent with the above-mentioned geometric shapes.
| Material | FWHM | Material | FWHM |
|---|---|---|---|
| ABNR | 0.03558 | ABNR-NH2 | 0.02165 |
| ZBNR | 0.03094 | ZBBR-NH2 | 0.02011 |
4. Conclusions
The molecular dynamics simulations suggest that Young’s moduli of the ABNR are slightly greater than those of the ZBNR. Grafting amine groups can increase moduli and decrease some anisotropy of the elastic moduli. Optimizing the structures of BNRs and BNRs-NH2 with different in-plane tensile strains shows that the critical elastic strain for the ABNR is less than the ZBNR and grafting amine groups can increase the strain. However, the ZBNR is somewhat different as grafting amine groups slightly reduces the strain. The result indicates that grafting amine groups can enhance the elastic strain range of the ABNR and efficiently reduce the anisotropy of elastic strain for the BNR. Their fracture modes are completely different; the ABNR and ZBNR show distinct typical ductile and brittle fractures, respectively. The crack for the ABNR begins from the middle and that for the ZBNR begins from both ends. The deformation process for the ABNR has four distinct stages: the linear elasticity, elastic-plastic, plastic and fracture phases, and the breakup of the ZBNR occurs so fast that one is unable to see the transitional processes. The analysis results for failure strain indicate that the main reason for the obvious cracking of the BNR is the bond tolerance; the grafting of amine groups is useful for strengthening the crack resistance of armchair but not zigzag conformations. Geometric structures of the BNRs and BNRs-NH2 at a quenching temperature of 1500 K show that the deformation of the armchair is slightly greater than that for the zigzag, but grafted amine groups can significantly strengthen the capacity to resist deformation at a high temperature and reduce the thermal expansion anisotropy. The analysis results for the RDF reveal that elastic properties, anti-deformability and high temperature structural stability of BNRs have some corresponding correlations with their crystallinities.Acknowledgements
The work described in this paper was supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. 9042047, CityU 11208914) and National Natural Science Foundation of China (Grant No. 51378448). The work was also supported by Hunan Provincial Natural Science Foundation of China (Project No. 14JJ2076), Aid Program for Teaching Reform Project of Changsha University of Science and Technology (Project No. JG1323), the Construct Program of the Key Discipline in Hunan Province and Aid Program for Science and the Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, China.References
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- Z. A. Piazza, H. S. Hu, W. L. Li, Y. F. Zhao, J. Li and L. S. Wang, Nat. Commun., 2014, 5, 3113 Search PubMed.
- W. L. Li, Q. Chen, W. J. Tian, H. Bai, Y. F. Zhao, H. S. Hu, J. Li, H. J. Zhai, S. D. Li and L. S. Wang, J. Am. Chem. Soc., 2014, 136, 12257–12260 CrossRef CAS PubMed.
- A. Chavez-Valdez, M. S. P. Shaffer and A. R. Boccaccini, J. Phys. Chem. B, 2013, 117, 1502–1515 CrossRef CAS PubMed.
- L. Xiaoyang, L. Peng, W. Yang, L. Qunqing, W. Jiaping, W. Yang, F. Chen, Z. Lina, F. Shoushan and J. Kaili, Nat. Commun., 2013, 4, 2920 Search PubMed.
- W. L. Li, Y. F. Zhao, H. S. Hu, J. Li and L. S. Wang, Angew. Chem., Int. Ed., 2014, 53, 5540–5545 CrossRef CAS PubMed.
- Q. Chen, G. F. Wei, W. J. Tian, H. Bai, Z. P. Liu, H. J. Zhai and S. D. Li, Phys. Chem. Chem. Phys., 2014, 16, 18282–18287 RSC.
- I. A. Popov, Z. A. Piazza, W.-L. Li, L.-S. Wang and A. I. Boldyrev, J. Chem. Phys., 2013, 139, 144307 CrossRef PubMed.
- W. Huang, A. P. Sergeeva, H. J. Zhai, B. B. Averkiev, L. S. Wang and A. I. Boldyrev, Nat. Chem., 2010, 2, 202–206 CrossRef CAS PubMed.
- I. Boustani, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 16426–16438 CrossRef CAS.
- F. Li, P. Jin, D. E. Jiang, L. Wang, S. B. Zhang, J. Zhao and Z. Chen, J. Chem. Phys., 2012, 136, 074302 CrossRef PubMed.
- S. De, A. Willand, M. Amsler, P. Pochet, L. Genovese and S. Goedecker, Phys. Rev. Lett., 2011, 106, 225502 CrossRef.
- I. Boustani, A. Quandt, E. Hernandez and A. Rubio, J. Chem. Phys., 1999, 110, 3176–3185 CrossRef CAS PubMed.
- J. Kunstmann and A. Quandt, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 035413 CrossRef.
- H. Tang and S. Ismail-Beigi, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 115412 CrossRef.
- Y. Ding, X. Yang and J. Ni, Appl. Phys. Lett., 2008, 93, 043107 CrossRef PubMed.
- T. R. Galeev, Q. Chen, J. C. Guo, H. Bai, C. Q. Miao, H. G. Lu, A. P. Sergeeva, S. D. Li and A. I. Boldyrev, Phys. Chem. Chem. Phys., 2011, 13, 11575–11578 RSC.
- E. S. Penev, S. Bhowmick, A. Sadrzadeh and B. I. Yakobson, Nano Lett., 2012, 12, 2441–2445 CrossRef CAS PubMed.
- H. G. Lu, Y. W. Mu, H. Bai, Q. Chen and S. D. Li, J. Chem. Phys., 2013, 138, 024701 CrossRef PubMed.
- S. Banerjee, G. Periyasamy and S. K. Pati, J. Mater. Chem. A, 2014, 2, 3856–3864 CAS.
- Y. Y. Liu, E. S. Penev and B. I. Yakobson, Angew. Chem., Int. Ed., 2013, 52, 3156–3159 CrossRef CAS PubMed.
- H. Tang and S. Ismail-Beigi, Phys. Rev. Lett., 2007, 99, 115501 CrossRef.
- X. B. Yang, Y. Ding and J. Ni, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 041402 CrossRef.
- X. J. Wu, J. Dai, Y. Zhao, Z. W. Zhuo, J. L. Yang and X. C. Zeng, ACS Nano, 2012, 6, 7443–7453 CrossRef CAS PubMed.
- H. Tang and S. Ismail-Beigi, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 134113 CrossRef.
- S. Er, G. A. de Wijs and G. Brocks, J. Phys. Chem. C, 2009, 113, 18962–18967 CAS.
- A. Hansson, M. Paulsson and S. Stafstrom, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 7639–7644 CrossRef CAS.
- D. Yu and F. Liu, Nano Lett., 2007, 7, 3046–3050 CrossRef CAS PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- J. H. Yuan and K. M. Liew, Carbon, 2009, 47, 713–721 CrossRef CAS PubMed.
- N. Yuxiang, J. Jiechao, E. Meletis and T. Dumitrica, Appl. Phys. Lett., 2015, 107, 031603 CrossRef PubMed.
- K. M. Liew and J. H. Yuan, Nanotechnology, 2011, 22, 085701 CrossRef CAS PubMed.
- J. H. Yuan and K. M. Liew, Phys. Chem. Chem. Phys., 2014, 16, 88–94 RSC.
- A. Montazeri, S. Ebrahimi and H. Rafii-Tabar, Mol. Simul., 2015, 41, 1212–1218 CrossRef CAS PubMed.
- R. Konnola, J. Joji, J. Parameswaranpillai and K. Joseph, RSC Adv., 2015, 5, 61775–61786 RSC.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. Goddard and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- N. Yao and V. Lordi, J. Appl. Physiol., 1998, 84, 1939–1943 CrossRef CAS PubMed.
- C. J. Casewit, K. S. Colwell and A. K. Rappe, J. Am. Chem. Soc., 1992, 114, 10035–10046 CrossRef CAS.
- L. Boldrin, F. Scarpa, R. Chowdhury and S. Adhikari, Nanotechnology, 2011, 22, 505702 CrossRef CAS PubMed.
- C. Lee, X. D. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- Z. H. Ni, H. M. Wang, J. Kasim, H. M. Fan, T. Yu, Y. H. Wu, Y. P. Feng and Z. X. Shen, Nano Lett., 2007, 7, 2758–2763 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of fullerenes and carbon nanotubes, Academic Press, San Diego, 1996 Search PubMed.
- E. Konstantinova, S. O. Dantas and P. Barone, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 035417 CrossRef.
- H. Zhao, K. Min and N. R. Aluru, Nano Lett., 2009, 9, 3012–3015 CrossRef CAS PubMed.
- G. B. Yadhukulakrishnan, S. Karumuri, A. Rahman, R. P. Singh, A. K. Kalkan and S. P. Harimkar, Ceram. Int., 2013, 39, 6637–6646 CrossRef CAS PubMed.
- M. Yoonessi, D. A. Scheiman, M. Dittler, J. A. Peck, J. Ilavsky, J. R. Gaier and M. A. Meador, Polymer, 2013, 54, 2776–2784 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |