Tuning SEI formation on nanoporous carbon–titania composite sodium ion batteries anodes and performance with subtle processing changes†
Abstract
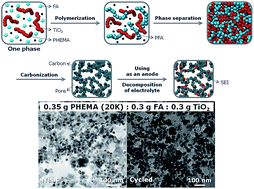
The morphology of composite materials used in battery electrodes is critical to provide the requisite transport paths for ions and electrons to enable high performance. In this work, we describe a simple and scalable method to fine tune the morphology of carbon/TiO2 composite through polymerization-induced phase separation of a mixture containing commercial TiO2 nanoparticles, poly(hydroxyethyl methacrylate) (PHEMA), and photoacid generator (PAG) dissolved in furfuryl alcohol (FA, monomer). UV exposure converts the PAG to a strong acid that catalyzes the FA polymerization to quickly initiate the polymerization. The morphology is modulated by the molecular weight of PHEMA and FA concentration that impact the miscibility and mobility during phase separation. The polymerized composite is carbonized to yield porous carbon/TiO2 electrodes. The cycling performance is dictated by the morphology that develops during phase separation. Electrochemical impedance spectroscopy (EIS) analysis illustrates that subtle changes in synthetic conditions can dramatically impact the electrical or ion conductance, primarily through modulation in the solid electrolyte interphase (SEI). A careful investigation of the SEI layer on the porous carbon/TiO2 composites demonstrates a clear correlation between the SEI and the surface area of the porous anode as determined by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). With selection of synthetic conditions to yield a modest surface area composite, sustainable anodes with stable capacity can be fabricated for use in Na ion batteries.

 Please wait while we load your content...
Please wait while we load your content...