The effect of the octan-3-yloxy and the octan-2-yloxy chiral moieties on the mesomorphic properties of ferroelectric liquid crystals†
Abstract
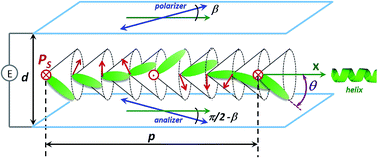
New mesogenic compounds exhibiting unique, so called orthoconic, behavior at the synclinic smectic SmC* phase have been obtained. The newly synthesized compounds belong to two chiral homologous series: 4′-[ω-(2,2,3,3,4,4,4-heptafluorobutoxy)alkoxy]biphenyl-4-yl-4-(octan-2-yloxy)benzoates and 4′-[ω-(butoxy)alkoxy]biphenyl-4-yl-4-(octan-3-yloxy)benzoates. Their mesogenic behavior has been studied and their phase transition temperatures as well as enthalpies have been evaluated using polarizing optical microscopy, differential scanning calorimetry and dielectric spectroscopy techniques. The tilt angle, the spontaneous polarisation as well as the helical pitch of the compounds have been studied in the full temperature domain. The compounds with a 4′-ω-(2,2,3,3,4,4,4-heptafluorobutoxy)alkoxy terminal chain exhibit a polar smectic C* phase. The analogous compound with a 4′-ω-(butoxy)alkoxy achiral terminal chain and an octan-2-yloxy chiral part exhibits an Iso-N*-SmC* phase sequence, while the one with an octan-3-yloxy chiral part does not exhibit mesogenic behavior. The compounds with the octan-3-yloxy chiral part have much lower melting points than those with the octan-2-yloxy chiral part. The clearing points of the new compounds decrease with the increase of the length of the oligomethylene spacer chain. Dielectric studies confirmed the presence of SmC* and N* phases. The tilt angles measured in the SmC* phase reveal extremely high values at saturation approaching 45°. The values of the spontaneous polarization for all investigated compounds are as high as 89.5 nC cm−2. The length of the helical pitch for different compounds varies from 460.7 nm to 1367.7 nm.

 Please wait while we load your content...
Please wait while we load your content...