Current trends and future prospects of lipstatin: a lipase inhibitor and pro-drug for obesity
Punit Kumar
and
Kashyap Kumar Dubey
*
Microbial Biotechnology Laboratory, University Institute of Engineering and Technology, Maharshi Dayanand University, Rohtak, Haryana, India. E-mail: punitdariyapur@gmail.com; kashyapdubey@gmail.com; Tel: +91-1262-272280
First published on 28th September 2015
Abstract
Obesity is an alarming state caused by an energy imbalance. It is associated with cardiovascular diseases, type-II diabetes, and cancer. Physical inactivity and overeating are the main causes of obesity but scientific reports also support the involvement of Single Nucleotide Polymorphisms (SNPs) in adiposity. Lipids are known to produce more energy than sugars and proteins, hence controlling the digestion of dietary lipids is a promising approach to treat obesity. Lipstatin is a β-lactone molecule which controls the digestive activity of pancreatic lipases and thus controls the absorption of fat in the small intestine. The three dimensional structure of pancreatic lipase revealed that its active site is masked by a lid domain which is opened by the action of bile salt micelles and colipase. Lipstatin is supposed to inhibit the catalytic activity of pancreatic lipase by acylation of the serine residue present in the active site. A binding study suggests that one molecule of lipstatin binds with one molecule of lipase. Tetra-hydro-lipstatin is an Active Pharmaceutical Ingredient (API) of lipstatin which is utilized in antiobesity medicines. Lipstatin is a natural product produced from Streptomyces toxytricini but poor productivity heightens the cost. The mechanism of lipstatin biosynthesis in bacteria is still unexplored. However, the acyl-coenzyme A carboxylase (ACCase) complex plays a key role in lipstatin biosynthesis. The present review focuses on the implications and causes of obesity, the status of antiobesity drugs, the mechanism of inhibition of pancreatic lipases, the biosynthesis of lipstatin and the present status of lipstatin production.
1.1 Introduction
Obesity is a physical condition in which fat accumulation adds more body weight to a healthy individual. Recent data from the World Health Organization (WHO)1 reveals that in 2014 about 1.9 billion people aged above 18 years were overweight and over 600 million of these were obese. Even in the United States of America (USA) more than 68% of adults are overweight2 and obesity is blamed for approximately 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths, and more than $147 billion in disease linked monetary expenses every year.3 Despite being a vexing problem of the world, obesity is not a health priority for developing countries such as India. Due to its large number of obese people and increasing cases of obesity, India may be suggested as the capital of diabetes and cardiovascular diseases.4 It is surprising that obesity and malnutrition coexist simultaneously in developing and under-developed countries and a large proportion of the global population resides in nations where overweight and obesity are responsible for more deaths than underweight.1 The WHO has recognized obesity as a disease of the 21st century.
000 deaths, and more than $147 billion in disease linked monetary expenses every year.3 Despite being a vexing problem of the world, obesity is not a health priority for developing countries such as India. Due to its large number of obese people and increasing cases of obesity, India may be suggested as the capital of diabetes and cardiovascular diseases.4 It is surprising that obesity and malnutrition coexist simultaneously in developing and under-developed countries and a large proportion of the global population resides in nations where overweight and obesity are responsible for more deaths than underweight.1 The WHO has recognized obesity as a disease of the 21st century.
1.1.1. Assessment of obesity
Body mass index (BMI) is a widely used method for obesity assessment. It is a mathematical ratio of weight (‘W’ in kilogram) and height (‘H’ in meters) and is calculated as W/H2. On the basis of BMI, individuals are classified as underweight, normal weight, overweight or obese (Table 1).5–8 Globally, a BMI above 25 denotes overweight and above 30 is obesity, but many nations have set different limits of BMI for obesity; for example, BMIs above 25 and 28 indicate obesity in Japan9 and China,10 respectively. Although BMI is a well recognized assessment of obesity, it does not always produce satisfactory results. Athletes may have higher BMI of up to 32 due to a large muscular mass. Two people may have different body shapes but the same BMI. Another method for obesity assessment is the measurement of waist circumference or waist/hip ratio, which is assumed to be a superior indicator of obesity to BMI11 and is assumed to predict abdominal fat, visceral fat and total fat in a better way. A waist circumference for women above 80 cm and above 94 cm for men is associated with obesity related risks. Other obesity assessment methods are imaging methods like magnetic resonance imaging and computed tomography which measure specific fat depots more precisely and accurately.11| BMI and its clinical significance5 | Waist circumference (cm) and level of health risk11 | |||
|---|---|---|---|---|
| BMI (kg m−2) | Clinical significance | Women | Men | Health risk |
| <18.50 | Underweight | <80 | <90 | Low |
| 18.50 to 24.99 | Normal weight | ≥94 to 101.9 | ≥80 to 87.9 | Increased |
| 25.00 to 29.99 | Overweight | ≥102 | ≥88 | High |
| 30.00 to 34.99 | Class I obesity (moderately obese) | |||
| 35.00 to 39.99 | Class II obesity (severely obese) | |||
| ≥40.00 | Class III obesity (very severely obese) | |||
It is well understood that the distribution of body fat also plays an important role in obesity associated complications. Individuals with more visceral and subcutaneous abdominal adipose tissue are found prone to obesity related health risks.7,12 However, visceral adipose tissues were found responsible for health risks than subcutaneous abdominal adipose tissue.13,14 Moreover, individuals looking “fit” and containing significantly more liver and visceral fat bear considerably more health risks of chronic disease.15
1.1.2 Diseases associated with obesity
Obesity is reported to form the foundation of many severe diseases such as type-2 diabetes, hypertension, coronary heart disease, non-alcoholic fatty liver disease, osteoarthritis, sleep apnea, cognitive decline, metabolic syndromes (blood lipid disorders, insulin resistance, inflammation, and cardiovascular diseases) and certain forms of cancer.7,16–19 Expression profile examination of adipose tissues revealed that adipose tissues express biologically active molecules, adipocytokines, which are directly associated with obesity linked diseases.201.1.3 Causes of obesity
The key reason for obesity is an imbalance between energy intake and energy expenditure, so extra energy gets stored in the body,21 but the patho-physiology of obesity is complex and not well elaborated. Obesity is due to an inactive lifestyle,22,23 stress and overeating but there are many other factors that induce overeating, reduce metabolic rate and overall cause obesity (Fig. 1). Hormonal imbalance between ghrelin and leptin due to lack of sleep24,25 and depression induces overeating.26 Endocrine disruptors, for example, diethylstilbestrol, polychlorinated bisphenyls, phthalate metabolites etc., disrupt the tasks of the endocrine system and are found to be associated with obesity.27–30 Health conditions like hypothyroidism,31 Cushing’s syndrome32 and polycystic ovarian syndrome33 are also reported to be associated with obesity.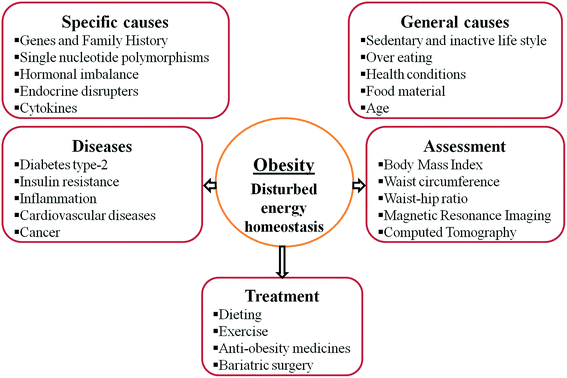 | ||
| Fig. 1 Various factors responsible for obesity, diseases associated with obesity, assessment of obesity and treatment options for obesity. | ||
Molecular analysis has revealed that gene polymorphism is associated with obesity. There are evidences that melanocortin-4 receptor mutations34 and TG polymorphism in the adiponectin gene35 have correlations with obesity. Single nucleotide polymorphism (SNP) variations near MC4R, especially rs1297034, are found to be associated with insulin resistance and waist circumference.36 Genome-wide association studies (GWAS) have recognized the linkage of approximately 40 SNPs with obesity, suggesting that multiple mechanisms are contributing towards obesity, and out of these, eight SNPs were confirmed by follow-up GWAS to be near or within genes (FTO, NEGR1, TMEM18, ETV5, FLJ35779, LINGO2, SH2B1 and GIPR) controlling appetite, enhancers of intracellular signalling and neural connections to energy balance.37 A twins study suggested that obesity is under genetic control and is inheritable.38 Families with a history of obesity, hypertension, or stroke were found to be prone to obesity and hyperlipidaemia and these problems may increase with age.39
1.1.4 Obesity and inflammation
Researchers have recognized the role of adipose tissue in the integration of metabolic, endocrine, and inflammatory signals for the control of energy homeostasis.40 The adipose tissues are reported to release chemoattractants (cytokines, interleukins, TNF-α etc.) which promote the infiltration of immune cells into the adipose tissue and induce an inflammatory response. This process is assumed to interrupt the insulin signaling cascade and glucose homeostasis which further causes metabolic disorders (insulin resistance and type-2 diabetes).41,42A balance between proinflammatory cytokines and anti-inflammatory cytokines is believed to aid healthy weight management, and an imbalance is thought to be associated with obesity. Researchers have found enhanced levels of the inflammatory mediator interleukin-6 and necrosis factor-alpha in obese individuals,43 and reduced levels in individuals with sustained weight loss.44 It is believed that a lack of physical activity, overnutrition, and aging induce cytokine hypersecretion which causes diabetes and resistance to insulin. Studies also support fat removal (liposuction) having positive effects on vascular inflammation and insulin resistance.45
1.2 Control of obesity
The control of obesity is achieved by keeping a balance between absorption and expenditure of energy. It is not necessary to maintain this balance daily but over time the existence of this balance maintains a healthy weight;| Ea > Ee = weight gain |
| Ea = Ee = weight balance |
| Ea < Ee = weight loss |
Although long term instruments for the prevention and control of obesity are leading to awareness, education and transformation of the obesogenic environment, treatment is also required for clinically obese individuals. Amazingly, for the high rate of prevalence, the treatment options are relatively inadequate.
The general recommendation for obesity management is a healthy lifestyle comprising diet management, physical work and exercises. Physical exercise is even recognized as the “exercise pill”.46–48 With this, antiobesity medicines and bariatric surgery are used to treat clinically obese patients.49 Antiobesity drugs are of two types: one reduces energy absorption and the other increases energy expenditure.50 For effective weight management, a combination of these approaches may be employed depending on the level of obesity, the existence of other diseases, the age of the person etc. Pharmacologically there are the following approaches for obesity control:51,52
(a) Reduction in digestion and absorption of high calorie molecules in the gastrointestinal tract.
(b) Reduction of food consumption by enhancing satiety, and decreasing appetite.
(c) Enhanced energy expenditure and controlling energy supply of adipose tissue.
(d) Decreasing inflammation of adipose tissues.
(e) Changing body fat distribution.
1.2.1. Complications of antiobesity approaches
The long-term effects of weight management approaches like diet regulation or exercise are not promising because of the absence of remarkable or sustainable weight loss. Dieting and increased physical activity are associated with weight regain.53 Bariatric surgery carries surgical risks, metabolic complications and high costs.54 Antiobesity medicines are good options but their efficacy and safety are questionable. These medicines are known to create side effects on the gastrointestinal system, kidneys, central nervous system and cardiovascular system, and many of the introduced antiobesity medicines have been withdrawn (for example sibutramine, rimonabant, contrive, and phentermine) or suspended because of safety concerns (Table 2). The United States Food and Drug Administration (FDA) approved orlistat, phendimetrazine, and diethylpropion, but only orlistat remains for long term use despite its moderate efficacy. In 2012, the FDA approved lorcaserin and phentermine–topiramate with acceptable side effects.18 One new molecule, cetilistat, which is reported to be more efficient and safe than orlistat, is currently in phase III clinical trials. Other molecules in clinical trials are gut-acting, such as exenatide and liraglutide.55 One new molecule has been reported by Finan et al., (2015) to target three receptors. The activity of this molecule has been confirmed in knocked-out mice but pharmacological studies remain to be done.56,57| Medicine | Remark | Mode of action | Drug target | Complications | Ref. |
|---|---|---|---|---|---|
| a AMPA: α-amino-3-hydroxyl-4-isoxazole-propionic acid, KA: kainate, EMEA: European Medicines Agency, FDA: US Food and Drug Administration. | |||||
| Aminorex | Introduced in 1965 in Europe and suspended in 1968 | Appetite suppression | Serotonin neurons | Pulmonary arterial hypertension, heart failure | 98 and 99 |
| Caffeine | Appetite suppression | Adenosine receptors | Nausea, vomiting, tachycardia, opisthotonos, myoclonic jerks, seizures, cerebral edema. Electrolyte abnormalities | 100 and 101 | |
| Cetilistat | Phase III clinical trial | Lipase inhibitor | Pancreatic lipase | Diarrhea, flatulence, bloating, abdominal pain, fatty stool | 55,96 and 102 |
| 2,4-Dinitrophenol | Introduced in 1933 and banned in 1938 | Increased fat metabolism | Mitochondrial oxidative phosphorylation | Hyperthermia, tachycardia, diaphoresis, tachypnea, cardiac arrest, cataract | 99,101 and 103 |
| Ephedra | Banned by FDA in 2004 | Appetite suppression | Adrenergic receptors | Myocardial infarction, hypertension, cardiac dysrhythmias, hemorrhagic and ischemic strokes | 101 |
| Fenfluramine dexfenfluramine | Approved by EMA in 1963 and FDA in 1973, suspended by FDA in 1997 | Appetite suppression | Serotonin (5-HT) neurons | Cardiac valve disease, primary pulmonary hypertension | 101,104 and 105 |
| Guar gum | Increased faecal excretion of lipids, satiety | Excretion of bile acids | Abdominal pain, flatulence, diarrhea, cramps | 101 and 106 | |
| Human chorionic gonadotropin | Changes fat distribution, preferentially burns stored fat, appetite suppression | Hypotension, hypoglycemia, constipation, fatigue | 101 and 107 | ||
| Ipecac | Approved in 1965 by FDA | Gastric emptying by vomiting | Gastric mucosal sensory receptor and central chemoreceptor trigger zone | Aspiration, Mallory–Weiss tears, pneumomediastinum, and intracerebral hemorrhage, reversible myopathy, complications of cardiovascular system | 101 and 108 |
| Laxatives | Electrolyte shift | Electrolytes | Chronic diarrhea, hypokalemia, melanosis coli, cathartic colon | 101 | |
| Lorcaserin | Approved in 2012 by FDA | Appetite suppression, decreased hunger | Serotonin 2C receptor | Headache, back pain, nasopharyngitis, nausea | 55 and 109 |
| Mazindol | Introduced in 1970 and suspended in 2000 | Appetite suppression | Serotonin neurons | Nervousness, atrial fibrillation, insomnia, syncope, pulmonary arterial hypertension | 99 |
| Orlistat | Approved by FDA in 1998 | Lipase inhibitor | Pancreatic lipase | Steatorrhoea, bloating, fecal incontinence, flatus, oily discharge, soft stools, adverse effect on gastrointestinal tract, kidney | 101,110 and 111 |
| Phentermine | Approved by FDA in 1959 | Reduction in food intake | β-Adrenergic receptors, norepinephrine, dopamine neurons | Dry mouth, headache, insomnia, nervousness, irritability, constipation, palpitations, tachycardia, and hypertension | 112 |
| Phenylpropanolamine | Introduced in 1939, banned by FDA in 2000 | Appetite suppression | α-Adrenergic receptors, catecholamine reuptake | Headache, tremor, insomnia, agitation, chest pain, palpitations, hypertension, hemorrhagic strokes, cerebral vasculitis, infarction | 101 and 113 |
| Rimonabant | Approved by EMEA in 2006, not approved by FDA, suspended in 2009 by EMEA | Reduced food intake | Cannabinoid type 1 receptor | Central nervous system, upper respiratory infections, nasopharyngitis, nausea, anxiety, depression, fatigue, dizziness | 101,114 and 115 |
| Sibutramine | Banned by FDA in 2004 | Appetite suppression, increased energy expenditure | Norepinephrine and serotonin reuptake inhibitor | Cardiovascular, hypertension, panic attacks and psychosis | 99,101 and 116 |
| Thyroid hormone | Increased basal metabolic rate | Centrally at hypothalamus | Hyperthyroidism, thyroid storm, thyrotoxic periodic paralysis | 101 and 117 | |
| Topiramate | Approved by FDA in 1996 | Increased energy expenditure, appetite suppressant | AMPA/KA receptor antagonism | Adverse effects on central and peripheral nervous system | 112 |
1.3 Role of lipids and lipid inhibitors in obesity
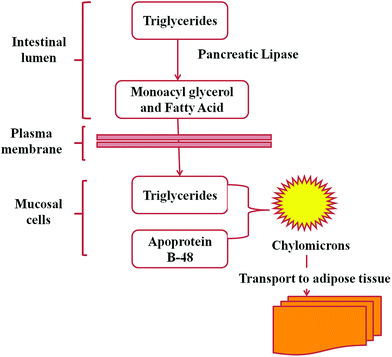
Lipids play very important roles in biological systems. They serve as the building blocks of phospholipids and glycolipids, and as the carriers of many proteins in cells, hormones and intracellular messengers. Lipids are stored as triacylglycerols (triglycerides) to work as fuel molecules which are formed from the esterification of fatty acids with glycerol.58Pancreatic lipases (a subclass of esterases) are PNLIP gene encoded enzymes that cause the hydrolysis of triacylglycerols (90–95% of dietary lipids)59 into fatty acids and monoacylglycerol (Fig. 2). The hydrolysed products are absorbed by the intestinal epithelium and resynthesized into triacylglycerol and then to chylomicrons which are transported to adipose tissues (Fig. 3).58 Triacylglycerols are reduced, anhydrous molecules and they serve as the concentrated storehouse of metabolic energy. The metabolic energy yield of a completely oxidised fatty acid is about 9 kcal g−1 (38 kJ g−1), compared to about 4 kcal g−1 (17 kJ g−1) for proteins and carbohydrates.58 Hence lipids are considered to be greatly responsible for obesity and thus the control of lipid digestion might be a very good strategy for obesity regulation. It is not easy to take a fat free diet but the digestion of fat may be regulated. Researchers are engaged in the search for efficient inhibitors of lipases to control lipid digestion and absorption in the intestine. This might be a striking approach, if it would decrease the risks of systemic side-effects.
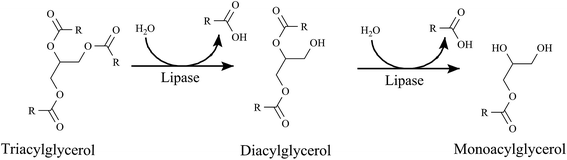 | ||
| Fig. 2 Hydrolysis of triacylglycerol by lipase. Lipase hydrolyses triacylglycerols into diacylglycerol and fatty acid and then monoacylglycerol and fatty acid. | ||
1.3.1 Natural lipase inhibitors
A large number of bacterial, fungal, algal and plant species have been screened for the presence of lipase inhibitors. Based on their chemical properties, isolated compounds are classified into different chemical groups such as terpenes, saponins, polyphenols, carotenoid, fucoxanthinol, lipstatin, esterastin, valilactone, panclicins, vibralactone, penicillamine derivatives and percyquinin. These compounds have plant, algal, bacterial and fungal origins (Table 3).| Origin | Name | Compound | References |
|---|---|---|---|
| Plants | Camellia sinensis | Polyphenols – proanthocyanidins and catechins, theasaponins | 118 |
| Cassia mimosoides | Polyphenols – proanthocyanidins | 119 | |
| Malus domestica | Polyphenols – procyanidins, catechins | 120 | |
| Arachis hypogaea | Polyphenols | 121 | |
| Nelumbo nucifera | Polyphenols | 122 | |
| Mangifera indica | Polyphenols | 123 | |
| Cassia nomame | Polyphenols – condensed tannins | 119 | |
| Salacia reticulata | Polyphenols – condensed tannins | 124 | |
| Glycine max | Polyphenols – isoflavones, diadzein | 125 | |
| Citrus unshiu | Polyphenols – flavonoids | 126 | |
| Ilex paraguariensis | Polyphenols – flavonoids | 127 | |
| Taraxacum officinale | Polyphenols | 128 | |
| Vitrus vinifera | Polyphenols – proanthocyanidins | 129 | |
| Coffea arabica | Saponins | 130 | |
| Aesculus turbinata | Saponins | 131 | |
| Dioscorea nipponica | Saponins | 132 | |
| Eleutherococcus senticosus | Saponins – triterpenoid | 133 | |
| Gardenia jasminoides | Saponins | 134 | |
| Gypsophila oldhamiana | Saponins | 135 | |
| Panax ginseng | Saponins – steroid | 136 | |
| Panax japonicas | Saponins | 137 | |
| Panax quinquefolium | Saponins | 138 | |
| Sapindus rarak | Saponins | 139 | |
| Tea Saponins | Saponins | 140 | |
| Aloe vera | Triterpenes | 141 | |
| Betula alba | Triterpenes | 142 | |
| Melissa officinalis | Triterpenes | 141 | |
| Origanum vulgare | Triterpenes | 141 | |
| Bacterial | Streptomyces sp. NR0619 | Panclicins | 143 |
| Streptomyces albolongus | Valilactone | 144 | |
| Streptomyces lavendulae strain MD4-C1 | Esterastin | 145 and 146 | |
| Streptomyces toxytricini | Lipstatin | 79 | |
| Algal | Caulerpa taxifolia | Caulerpenyne | 147 |
| Caulerpa prolifera | Caulerpenyne | 147 | |
| See weeds | Fucoxanthinol (carotenoid) | 148 | |
| Fungal | Phellinus linteus | 149 | |
| Boreostereum vibrans | Vibralactone | 150 | |
| Monascus sp. | Penicillamine derivative | 151 | |
| Stereum complicatum, ST 001837 | Percyquinin | 152 |
Aside from these compounds, many other compounds have also been reported to possess lipase inhibitory properties such as chitosan oligosaccharides, water soluble chitosan, polydextrose, phytic acid, myoinositol phosphate esters, phenylboronic acid, carnosic acid, diterpenes, platycodin D, dioscin etc.59,60
2.1 Lipstatin
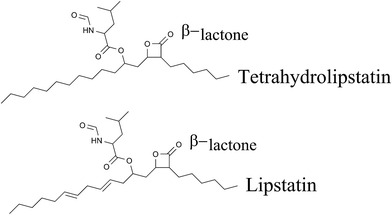
A large number of natural lipase inhibitors have been isolated and characterized. Lipstatin is one of these and is used commercially to synthesize antiobesity medicine. Lipstatin exhibits structural similarity to another microbial compound, the esterase inhibitor esterastin. A spectroscopic and chemical study proposed the molecular formula of lipstatin as C29H45NO5 and the structure as (2S,3S,5S,7Z,10Z)-5-[(S)-2-formamido-4-methylpentanoyloxy]-2-hexyl-3-hydroxy-7,10-hexadecadienoic lactone.61 Lipstatin has one β-lactone ring, and two aliphatic side chains with chain lengths of 6 and 13 carbon atoms. One side chain contains two isolated double bonds and one hydroxy group esterified to N-formyl-leucine.622.1.1 Biosynthesis of lipstatin
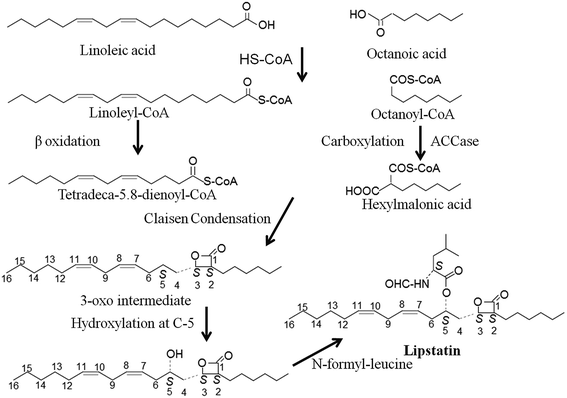
The exact molecular mechanism of lipstatin synthesis is still unknown but a chemical synthesis using radiolabeled lipid mixtures suggested that Claisen condensation takes place between two fatty acid precursors (octanoic acid and 3-hydroxy-tetradeca-5.8-dienoic acid). These precursors are produced by fatty acid metabolism and on condensation they create the β-lactone moiety of lipstatin.63,64Through molecular study researchers have discovered the involvement of the acyl-coenzyme A carboxylase (ACCase) complex in lipstatin biosynthesis. The ACCase complex is involved in the biosynthesis of fatty acids and polyketides by the catalytic activation of organic acids.65
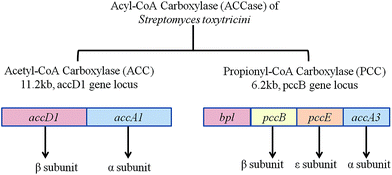
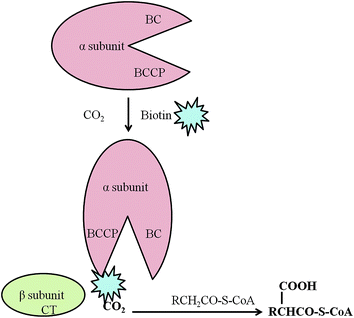
Two gene clusters for the ACCase complex are reported in Streptomyces toxytricini. One gene cluster, accA1, encodes for the α and β subunits of the acetyl-coenzyme A carboxylase complex (ACC) and another gene cluster pccB encodes for the α, β, and ε subunits and biotin protein ligase (Bpl) domain of the propionyl-coenzyme A carboxylase (PCC) complex (Fig. 4). The β subunit and ε subunit form an active protein complex. The α subunit contains a biotin carboxyl carrier protein (BCCP) domain and biotin carboxylase (BC) domain for biotin attachment and fixing of CO2 onto the biotin. The β subunit contains a carboxyltransferase domain (CT) that catalyses the transfer of the carboxyl group to acyl-coenzyme A (Fig. 5). Gene disruption by a PCR targeting system66,67 explained that ACC is involved in the primary metabolism of fatty acid synthesis and PCC regulates the secondary metabolism of polyketide biosynthesis. Although a direct association of the ACC and PCC complexes of Streptomyces toxytricini in lipstatin biosynthesis was not found, inactivation by disruption of the pccB and pccE genes resulted in the accumulation of tetradeca-5.8-dienoic acid and an 80% reduction of lipstatin biosynthesis. Hence it was suggested that the activation of the lipstatin precursor octanoic acid into hexylmalonic acid is regulated by the pccB of Streptomyces toxytricini. However, lipstatin synthesis occurred to a lesser degree, and this may be due to the activity of another ACC complex with lower activity. In addition, there was an accumulation of tetradeca-5.8-dienoic acid instead of 3-hydroxy-tetradeca-5.8-dienoic acid63 in the disruptant which suggested the idea that Claisen condensation takes place between tetradeca-5.8-dienoic acid and hexymelonic acid and there is the formation of a 3-oxo intermediate (Fig. 6). After this intermediate formation, one hydroxyl group is introduced at the C-5 position.65,68,69 Recently, a group of researchers has reported the involvement of a 3-hydroxysteroid dehydrogenase-homologous gene in the β-keto group reduction of the intermediate and the synthesis of the β-lactone structure.70
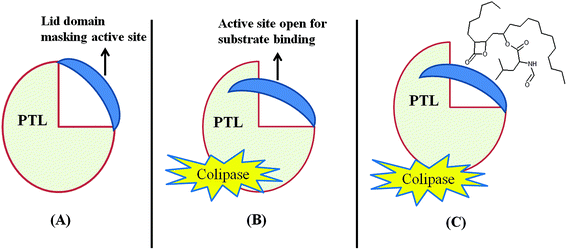
2.1.2 Structure of pancreatic lipase and inhibition of activity by tetrahydrolipstatin
Pancreatic triglyceride lipase (PTL) belongs to the lipase gene family and requires a colipase cofactor for enzymatic activity. PTL also possesses a high degree of structural and sequence homology with two other exocrine proteins, pancreatic lipase related proteins PLRP1 and PLRP2.71 The 3D structure revealed that PTL contains two distinct domains: one N-terminal domain composed of residues 1 to 336 and another C-terminal domain composed of residues 337 to 449.72 The amino acids present in the N-terminal domain, Ser 153, Asp 177 and His 163, make up the active site73,74 and the colipase binding site at the C-terminal.75 One lid domain formed by Cys 238 and Cys 262 connected by a disulphide bridge interacts with the β5 loop and β9 loop which mask the active site during the inactivated conformation state.74 The lid domain and β5 loop attain new conformations upon activation by bile salt micelles, colipase and the lipid water interface, and the active site is opened (Fig. 7). After this lid movement a large hydrophobic plateau of over 50 Å is created by colipase and PTL interaction, to interact with the lipid water interface.72 It was also found that bile salt micelles are able to open the lid in aqueous solution with or without colipase. The expression of PLRP1, PLRP2 and PTL takes place in exocrine pancreas cells but the amount of expression of each varies with different species and age.72 The binding of β-lactone to lipase has still not been studied but it has been found that tetrahydrolipstatin (THL) binds to PTL in the presence of bile salt micelles and colipase,76 hence THL is assumed to bind at the active site of PTL. Beside this it is believed that the β-lactone ring of lipstatin/THL somehow causes acylation of the serine hydroxyl site present in the active site, thus making the lipase catalytically inactive.77A binding study of 3H THL to lipase and bovine serum albumin revealed that THL binds to lipase specifically and forms a covalent complex with it. It was also assumed that one molecule of THL was sufficient to stop the lipolytic activity of one molecule of lipase and the stoichiometry of this reaction is near to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Side by side lipase inhibitory actions of THL increase with time and with increasing concentration of THL. The triolein substrate and transition state formation inhibitor boronic acid reduced the rate of inhibition of lipase by THL and also revealed that THL binds at or near the active site of lipase. THL is soluble (entirely distributed) in the presence of the substrate (lipid phase), hence inhibition is dependent on the lipid phase. It was also found that lipstatin inhibits PTL specifically while the activity of trypsin, chymotrypsin, esterase (liver), phospholipase A2, and α-amylase was not inhibited at 200 μM concentration.78
1. Side by side lipase inhibitory actions of THL increase with time and with increasing concentration of THL. The triolein substrate and transition state formation inhibitor boronic acid reduced the rate of inhibition of lipase by THL and also revealed that THL binds at or near the active site of lipase. THL is soluble (entirely distributed) in the presence of the substrate (lipid phase), hence inhibition is dependent on the lipid phase. It was also found that lipstatin inhibits PTL specifically while the activity of trypsin, chymotrypsin, esterase (liver), phospholipase A2, and α-amylase was not inhibited at 200 μM concentration.78
Lipstatin and THL cause dose dependent inhibition of PTL. The IC50 of an inhibitory assay of lipstatin was reported to be 0.14 μM. A dose dependent inhibition assay also confirmed the involvement of β-lactone in PTL activity inhibition as the opening of the β-lactone of lipstatin was ineffective at more than 2000 μM concentration.79
2.1.3 Production of lipstatin
Lipstatin is synthesized by the actinobacteria Streptomyces toxytricini and Streptomyces virginiae.71 According to the existing taxonomic principles, Streptomyces toxytricini belongs to the Streptomyces lavendulae phenotypic cluster.80 Initial reports of the fermentative isolation of this compound were submitted by Weibel et al., (1987)79 and its structural elucidation through NMR and IR spectroscopic methods was performed by Hochuli et al., (1987)61 at Hoffman La-Roche & Co. Switzerland.The chemical synthesis of lipstatin has been attempted81 by multistep synthesis but the yield was 38%, making it high cost. Hence the production of lipstatin through bacterial fermentation processes is found to be more economical and commercially viable.
The initial experiment produced 43.17 μg g−1 lipstatin.79 Improvement in lipstatin production mainly depends on the selection and development of high productivity strains and fermentation process conditions (culture medium and culture conditions) i.e. the optimization of these two complementary aspects. After optimization of the experimental conditions, by changing factors which require the use of statistical tools, like central composite design and response surface methodology, researchers have reported higher concentrations of lipstatin of 885 μg ml−1,82 1980 μg ml−1,62 3290 μg ml−1 (ref. 83) and 4208 μg ml−1.84 By looking into the depth of available technology, there is always a chance of obtaining a better titer which is suitable for commercial exploitation.
2.1.4 Clinical usage of lipstatin
Lipstatin is hydrogenated in order to synthesize the saturated molecule tetrahydrolipstatin, which is marketed as an antiobesity medicine with the name orlistat (Xenical, Fig. 8). Lipase inhibitory assays show that THL has a higher IC50 of 0.36 μM (ref. 79) in comparison to lipstatin (IC50 = 0.14 μM) but regardless of its lesser activity, the stability of THL has gained more attention in synthetic studies.81 Orlistat was found to inhibit about 30% of lipid absorption in the intestine.85,86 It also inhibits the absorption of fat soluble vitamins, for which oral supplemental vitamins are recommended.87Orlistat is a poorly water soluble molecule, and solubility plays an important role in the absorption of medicine and thereby its curative effect. Researchers have used solid dispersion methods using solvent evaporation techniques to enhance the solubility of orlistat.88
Orlistat was granted FDA approval in 1998.89 In clinical trials orlistat was found to be beneficial in reducing body weight by 5–10% in 50–60% of patients and this weight loss was maintained for up to four years.51 In one experiment, orlistat was found to lead to weight loss of up to 2.89 kg in 12 months.90 Orlistat treatment was also found to be beneficial for controlling BMI, cardiovascular risk, blood pressure, type-2 diabetes, and glycaemia, and improved LDL-C in obese patients with CAD and hypercholesterolemia.91–94
The clinical usage of orlistat is increasing continuously. Because of this, the demand for lipstatin from pharmaceutical industries is increasing and thus there is always a requirement to obtain the highest possible yield of lipstatin. Therefore, there is a focus on biochemical routes for the production of lipstatin through fermentation with high productivity strains, genetic make-up changes and studies on enhancing the bacterial productivity so that commercial/economical production of lipstatin might be cost effective.
2.1.5 Side effects of orlistat
Orlistat may be considered an ideal medicine for obesity but it is not free of side effects. However, in comparison to other drugs its side effects are limited. The FDA issued a communication regarding the safety aspects of orlistat in August 2009. Orlistat causes unpleasant side effects like oily stools, diarrhoea, abdominal pain, dyspepsia, faecal spotting, steatorrhoea (fecal fat loss) and some hepatic toxicity effects.95 Orlistat was also found to be associated with side-effects on the gastrointestinal tract and on the kidneys.963.1 Future prospects
Over the last two decades, the global occurrences of being overweight and obese have increased enormously, but there has been insufficient advancement in the discovery and development of new anti-obesity molecules with high efficacy and acceptable side effects. Besides this, a limited fraction of obese patients are treated via pharmacotherapy due to the narrow effectiveness of existing drugs and high cost. Regulatory agencies (FDA, EU) of drug approval have withdrawn many anti-obesity drugs (sibutramine, rimonabant, contrive, and phentermine) which had various adverse side effects on obese people.51,55It is believed that the market size for anti-obesity drugs will increase from $750 million in 2012 to $2.6 billion in 2019 (ref. 97) and orlistat forms the largest proportion of consumed anti-obesity drugs.
Streptomyces toxytricini and Streptomyces virginiae79,80 are well known viable sources for lipstatin production but poor productivity is a major concern, hence scientists must isolate alternative high yielding lipstatin producing strains from natural sources.
However, scientific data supports the enhancement of lipstatin production up to 4208 μg ml−1 (ref. 84) by optimizing fermentation parameters, media engineering, and changing upstream and downstream processing factors. Thus a further increase in lipstatin production is considerably challenging for researchers. It is evident that without a complete investigation of the functional genomics of lipstatin biosynthesis, further improvement is not feasible. Although the involvement of ACCase in lipstatin biosynthesis has been elucidated,65,68,69 full proof and experimental evidence are needed to explain the role of the various precursors, reaction intermediates, participatory enzymes and cofactors involved in lipstatin biosynthesis. Researchers must explore a metabolic engineering approach for strain development, though it is dependent on the knowledge of metabolomics, genomics, transciptomics, flux investigation, and efficient genetic tools.
It is well known that lipstatin biosynthesis is a cascade reaction process and researchers must find out the rate limiting steps. Along with the impact of precursor availability, strain development using genetic engineering approaches employing directed evolution, site-specific mutation and the combination of mutations must be analyzed to enhance the production of lipstatin by optimizing the activity of key enzymes individually and in combination.
Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.Acknowledgements
The authors sincerely acknowledge the Science and Engineering Research Board, (Department of Science and Technology, Govt. of India, SR/FT/LS-45/2012) for providing financial assistance and Maharshi Dayanand University Rohtak, Haryana, India for providing the necessary facilities for this research work.References
- WHO media center, Obesity and overweight, http://www.who.int/mediacentre/factsheets/fs311/en/, accessed on 02-06-15.
- S. M. Wright and L. J. Aronne, Abdom. Organ Imaging, 2012, 37, 730–732 CrossRef PubMed.
- E. A. Finkelstein, J. G. Trogdon, J. W. Cohen and W. Dietz, Health Aff., 2009, 28, 822–831 CrossRef PubMed.
- A. G. Unnikrishnan, S. Kalra and M. K. Garg, Indian J. Endocrinol. Metab., 2012, 16, 4–6 Search PubMed.
- BMI classification. World Health Organization, http://apps.who.int/bmi/index.jsp?introPage=intro_3.html, accessed on 02-06-15.
- D. H. Bessesen, J. Clin. Endocrinol. Metab., 2008, 93, 2027–2034 CrossRef CAS PubMed.
- J. P. Despres and I. Lemieux, Nature, 2006, 444, 881–887 CrossRef CAS PubMed.
- S. M. Grundy, J. I. Cleeman and S. R. Daniels, Circulation, 2005, 112, 2735–2752 CrossRef PubMed.
- M. Kanazawa, N. Yoshiike, T. Osaka, Y. Numba, P. Zimmet and S. Inoue, World Rev. Nutr. Diet., 2005, 94, 1–12 Search PubMed.
- B. F. Zhou, Cooperative Meta-Analysis Group of the Working Group on Obesity in China, Biomed. Environ. Sci., 2002, 15, 83–96 Search PubMed.
- T. S. Han, N. Sattar and M. Lean, Br. Med. J., 2006, 333, 695–698 CrossRef PubMed.
- C. S. Fox, J. M. Massaro and U. Hoffmann, et al., Circulation, 2007, 116, 39–48 CrossRef PubMed.
- S. A. Porter, J. M. Massaro, U. Hoffmann, R. S. Vasan, C. J. O’donnel and C. S. Fox, Diabetes Care, 2009, 32, 1068–1075 CrossRef PubMed.
- J. L. Kuk, P. T. Katzmarzyk, M. Z. Nichaman, T. S. Church, S. N. Blair and R. Ross, Obesity, 2006, 14, 336–341 CrossRef PubMed.
- G. O’Donovan, E. L. Thomas, J. P. McCarthy, J. Fitzpatrick, G. Durighel, S. Mehta, S. Morin, A. P. Goldstone and J. D. Bell, Int. J. Obes., 2009, 33, 1356–1362 CrossRef PubMed.
- D. W. Haslam and W. P. James, Lancet, 2005, 366, 1197–1209 CrossRef.
- National Institutes of Health and National Heart Lung and Blood Institute, What Are the Health Risks of Overweight and Obesity? http://www.nhlbi.nih.gov/health/health-topics/topics/obe/risks, accessed on 02-06-2015.
- A. J. Scheen and L. F. V. Gaal, Lancet Diabetes Endocrinol., 2014, 2(11), 911–922 CrossRef CAS.
- E. E. Calle and M. J. Thun, Oncogene, 2004, 23, 6365–6378 CrossRef CAS PubMed.
- Y. Matsuzawa, I. Shimomura, S. Kihara and T. Funahashi, Horm. Res., 2003, 60(suppl. 3), 56–59 CrossRef CAS PubMed.
- S. Bleich, D. Cutler, C. Murray and A. Adams, Annu. Rev. Public Health, 2008, 29, 273–295 CrossRef PubMed.
- R. R. Wing, M. G. Goldstein, K. J. Acton, L. L. Birch, J. M. Jakicic, J. R. J. F. Sallis, D. Smith-west, R. W. Jeffery and R. S. Surwit, Diabetes Care, 2001, 24, 117–123 CrossRef CAS.
- A. S. Barnes, Obesity and Sedentary Lifestyles, 2012, vol. 39, pp. 224–227 Search PubMed.
- S. Taheri, L. Lin, D. Austin, T. Young and E. Mignot, PLoS Med., 2004, 1(3), e62 CrossRef PubMed.
- K. E. Wortley, J. P. del Rincon, J. D. Murray, K. Garcia, K. Iida, M. O. Thorner and M. W. Sleeman, J. Clin. Invest., 2005, 115, 3573–3578 CrossRef CAS PubMed.
- X. Y. Lu, Curr. Opin. Pharmacol., 2007, 7, 648–652 CrossRef CAS PubMed.
- P. F. Baillie-Hamilton, Journal of Alternative and Complementary Medicine, 2002, 8, 185–192 CrossRef PubMed.
- J. J. Heindel, Toxicol. Sci., 2003, 76, 247–249 CrossRef CAS PubMed.
- M. A. Elobeid and D. B. Allison, Curr. Opin. Endocrinol., Diabetes Obes., 2008, 15, 403–408 CrossRef CAS PubMed.
- R. R. Newbold, Hormones, 2010, 9, 206–217 CrossRef.
- B. J. Hoogwerf and F. Q. Nuttall, Am. J. Med., 1984, 76, 963–970 CrossRef CAS.
- O. Tiryakioglu, S. Ugurlu, S. Yalin, S. Yirmibescik, E. Caglar, D. O. Yetkin and P. Kadioglu, Clinics, 2010, 65, 9–13 CrossRef PubMed.
- S. Sam, Obesity Management, 2007, 3, 69–73 CrossRef PubMed.
- C. Vaisse, K. Clement, E. Durand, S. Hercberg, B. Guy-Grand and P. Froguel, J. Clin. Invest., 2000, 106, 253–262 CrossRef CAS PubMed.
- M. Stumvoll, O. Tschritter, A. Fritsche, H. Staiger, W. Renn, M. Weisser, F. Machicao and H. Haring, Diabetes, 2002, 51, 37–41 CrossRef CAS.
- J. C. Chambers, P. Elliott, D. Zabaneh, W. Zhang, Y. Li, P. Froguel, D. Balding, J. Scott and J. S. Kooner, Nat. Genet., 2008, 40, 716–718 CrossRef CAS PubMed.
- J. R. Speakman, Hum. Hered., 2013, 75, 57–79 CrossRef CAS PubMed.
- A. Stunkard, T. Foch and Z. Hrubec, JAMA, 1986, 256, 51–54 CrossRef CAS PubMed.
- M. A. B. van der Sande, G. E. L. Walraven, P. J. M. Milligan, W. A. S. Banya, S. M. Ceesay, O. A. Nyan and K. P. W. J. McAdam, Bull. W. H. O., 2001, 79, 321–328 CAS.
- A. Behn and E. Ur, Current Opinion in Cardiology, 2006, 21, 353–360 CrossRef PubMed.
- G. S. Hotamisligil, Nature, 2006, 444, 860–867 CrossRef CAS PubMed.
- M. A. McArdle, O. M. Finucane, R. M. Connaughton, A. M. McMorrow and H. M. Roche, Front. Endocrinol., 2013 DOI:10.3389/fendo.2013.00052.
- T. U. Ciftci, O. Kokturk, N. Bukan and A. Bilgihan, Cytokine, 2004, 28, 87–91 CrossRef CAS PubMed.
- P. Ziccardi, F. Nappo, G. Giugliano, K. Esposito, R. Marfella, M. Cioffi, F. D’Andrea, A. M. Molinari and D. Giugliano, Circulation, 2002, 105, 804–809 CrossRef CAS PubMed.
- K. Esposito, G. Giugliano, N. Scuderi and D. Giugliano, Plast. Reconstr. Surg., 2006, 118, 1048–1059 CrossRef CAS PubMed.
- L. J. Goodyear, N. Engl. J. Med., 2008, 359, 1842–1844 CrossRef CAS PubMed.
- J. Himms-Hagen, Curr. Drug Targets: CNS Neurol. Disord., 2004, 3, 389–409 CrossRef CAS.
- V. A. Narkar, Cell, 2008, 134, 405–415 CrossRef CAS PubMed.
- M. J. F. Bult, T. van Dalen and A. F. Muller, Eur. J. Endocrinol., 2008, 158, 135–145 CrossRef CAS PubMed.
- A. Chatzigeorgiou, E. Kandaraki, A. G. Papavassiliou and M. Koutsilieris, Obes. Rev., 2014, 15, 487–503 CrossRef CAS PubMed.
- R. F. Witkamp, Pharm. Res., 2011, 28, 1792–1818 CrossRef CAS PubMed.
- J. C. G. Halford, E. J. Boyland, J. E. Blundell, T. C. Kirkham and J. A. Harrold, Nat. Rev. Endocrinol., 2010, 6, 255–269 CrossRef CAS PubMed.
- D. C. Lau, J. D. Douketis, K. M. Morrison, I. M. Hramiak, A. M. Sharma and E. Ur, CMAJ, 2007, 176, 1–13 Search PubMed.
- J. Couzin, Science, 2008, 320, 438–440 CrossRef CAS PubMed.
- M. George, M. Rajaram and E. Shanmugam, J. Cardiovasc. Pharmacol. Ther., 2014, 19(1), 65–76 CrossRef CAS PubMed.
- B. Finan, et al., Nat. Med., 2015, 21, 27–36 CrossRef CAS PubMed.
- A. J. Scheen and N. Paquot, Nat. Rev. Endocrinol., 2015, 11, 196–198 CAS.
- J. M. Berg, J. L. Tymoczko and L. Stryer, Biochemistry, W. H. Freeman and Company, New York, 5th edn, 2002, ch. 22 Search PubMed.
- A. L. de la Garza, F. I. Milagro, N. Boque, J. Campión and J. A. Martínez, Planta Med., 2011, 77, 773–785 CrossRef CAS PubMed.
- S. Singh and K. S. Singh, Int. Res. J. Med. Sci., 2013, 1, 15–26 Search PubMed.
- E. Hochuli, E. Kupfer, R. Maurer, W. Meister, Y. Mercadal and K. Schmidt, J. Antibiot., 1987, 40, 1086–1091 CrossRef CAS.
- M. S. Kumar, V. Verma, S. Soni and A. S. P. Rao, Adv. Biotechnol., 2012, 11, 06–11 Search PubMed.
- W. Eisenreich, E. Kupfer, W. Weber and A. Bacher, J. Biol. Chem., 1997, 10, 867–874 CrossRef PubMed.
- M. Goese, W. Eisenreich, E. Kupfer, P. Stohler, W. Weber, H. G. Leuenberger and A. Bacher, J. Org. Chem., 2001, 66, 4673–4678 CrossRef CAS PubMed.
- A. V. Demirev, A. Khanal, N. P. K. Hanh, K. T. Nam and D. H. Nam, J. Microbiol., 2011, 49, 407–412 CrossRef CAS PubMed.
- E. Rodriguez and H. Gramajo, Microbiology, 1999, 145, 3109–3119 CrossRef CAS PubMed.
- L. Diacovich, S. Peiru, D. Kurth, E. Rodriguez, F. Podesta, C. Khosla and H. Gramajo, J. Biol. Chem., 2002, 277, 31228–31236 CrossRef CAS PubMed.
- A. V. Demirev, A. Khanal, B. R. Sedai, S. K. Lim, M. K. Na and D. H. Nam, Appl. Microbiol. Biotechnol., 2010, 87, 1129–1139 CrossRef CAS PubMed.
- A. V. Demirev, J. S. Lee, B. R. Sedai, I. G. Ivanov and D. H. Nam, J. Microbiol., 2009, 47, 473–478 CrossRef CAS PubMed.
- T. Bai, D. Zhang, S. Lin, Q. Long, Y. Wang, H. Ou, Q. Kang, Z. Deng, W. Liu and M. Tao, Appl. Environ. Microbiol., 2014, 80, 7473–7483 CrossRef CAS PubMed.
- M. E. Lowe, Biochimie, 2000, 82, 1–8 CrossRef.
- M. E. Lowe, J. Lipid Res., 2002, 43, 2007–2016 CrossRef CAS.
- F. Faustinella, L. C. Smith, C. F. Semenkovich and L. Chan, J. Biol. Chem., 1991, 266, 9481–9485 CAS.
- F. K. Winkler, A. D’Arcy and W. Hunziker, Nature, 1990, 343, 771–774 CrossRef CAS PubMed.
- H. van Tilbeurgh, M. P. Egloff, C. Martinez, N. Rugani, R. Verger and C. Cambillau, Nature, 1993, 162, 814–820 CrossRef PubMed.
- Q. Luthi-Peng and F. K. Winkler, Eur. J. Biochem., 1992, 205, 383–390 CrossRef CAS PubMed.
- A. K. Al-Suwailem, A. S. Al-Tamimi, M. A. Al-Omar and M. S. Al-Suhibani, J. Appl. Sci. Res., 2006, 2, 205–208 Search PubMed.
- P. Hadvary, H. Lengsfeld and H. Wolfer, Biochem. J., 1988, 256, 357–361 CrossRef CAS.
- E. K. Weibel, P. Hadvary, E. Hochuli, E. Kupfer and H. Lengsfeld, J. Antibiot., 1987, 40(8), 1081–1085 CrossRef CAS.
- G. Sladic, M. Urukalo, M. Kirn, U. Lesnik, V. Magdevska, N. Benicki, M. Pelko, A. Gasparic, P. Raspor, T. Polak, S. Fujs, P. A. Hoskisson and H. Petkovic, Food Technol. Biotechnol., 2014, 52, 276–284 CAS.
- A. Pommier, J. M. Pons and P. J. Kocienski, J. Org. Chem., 1995, 60, 7334–7339 CrossRef CAS.
- U. Luthra and R. C. Dubey, Int. J. Microbiol. Res., 2012, 4, 266–269 CrossRef.
- U. Luthra, H. Kumar, N. Kulshreshtha, A. Tripathi, A. Trivedi, S. Khadpekar, A. Chaturvedi and R. C. Dubey, Nat. Sci., 2013, 11, 73–76 Search PubMed.
- T. Zhu, L. Wang, W. Wang, Z. Hu, M. Yu, K. Wang and Z. Cui, J. Gen. Appl. Microbiol., 2014, 60, 106–111 CrossRef CAS.
- B. Borgstrom, Biochim. Biophys. Acta, 1988, 962, 308–316 CrossRef CAS.
- J. Hauptman, C. Lucas, M. N. Boldrin, H. Collins and K. R. Segal, Arch. Fam. Med., 2000, 9, 160–167 CrossRef CAS PubMed.
- N. Finer, W. P. James, P. G. Kopelman, M. E. Lean and G. Williams, Int. J. Obes., 2000, 24, 306–313 CrossRef CAS PubMed.
- R. Bhimavarapu, K. P. Chitra, M. Anne, K. Dhavani, M. Haritha and N. Gowthami, Int. J. Biomed. Adv. Res., 2011, 2, 435–443 Search PubMed.
- R. J. Rodgers, M. H. Tschöp and J. P. H. Wilding, Dis. Models & Mech., 2012, 5, 621–626 CAS.
- Z. Li, M. Maglione, W. Tu, W. Mojica, D. Arterburn, L. R. Shugarman, L. Hilton, M. Suttorp, V. Solomon, P. G. Shekelle and S. C. Morton, Ann. Intern. Med., 2005, 142, 532–546 CrossRef CAS.
- S. B. Heymsfield, K. R. Segal, J. Hauptman, C. P. Lucas, M. N. Boldrin and A. Rissanen, Arch. Intern. Med., 2000, 160, 1321–1326 CrossRef CAS PubMed.
- I. Broom, J. Wilding, P. Scott and N. Myers, Int. J. Clin. Pract., 2002, 56, 494–499 CAS.
- J. S. Torgerson, J. Hauptman, M. N. Boldrin and L. Sjostrom, Diabetes Care, 2004, 27, 155–161 CrossRef CAS.
- K. W. Chan, W. S. Leung, Y. S. Fung, H. F. Hung, P. Tsui, H. Chu, Y. W. Chan, G. T. Ko and V. T. Yeung, Ir. J. Med. Sci., 2009, 178, 173–178 CrossRef CAS PubMed.
- G. A. Bray and F. L. Greenway, Pharmacol. Rev., 2007, 59, 151–184 CrossRef CAS PubMed.
- P. Kopelman, H. Groot Gde, A. Rissanen, S. Rossner, S. Toubro, R. Palmer, R. Hallam, A. Bryson and R. Hickling, Obesity, 2010, 18, 108–115 CrossRef CAS PubMed.
- GBI Reseach, Anti-obesity Drug Market to Increase by 2019, but Stigma Attached to Treatment Still a Major Hurdle, http://gbiresearch.com/media-center/press-releases/antiobesity-drug-market-to-increase-by-2019-but-stigma-attached-to-treatment-still-a-major-hurdle, accessed on 06-06-15.
- H. P. Gurtner, Cor Vasa, 1985, 27, 160–171 CAS.
- C. S. Elangbam, Vet. Pathol., 2009, 46(1), 10–24 CrossRef CAS PubMed.
- A. M. Dietrich and M. E. Mortensen, Pediatr. Emerg. Care, 1990, 6, 296–298 CrossRef CAS.
- M. Yen and M. B. Ewald, J. Med. Toxicol., 2012, 8, 145–152 CrossRef CAS PubMed.
- B. M. Y. Cheung, T. T. Cheung and N. R. Samaranayake, Ther. Adv. Drug Saf., 2013, 4, 171–181 CrossRef PubMed.
- W. W. Boardman, Calif. West. Med., 1935, 43, 118–119 CAS.
- H. M. Connolly, J. L. Crary, M. D. McGoon, D. D. Hensrud, B. S. Edwards, W. D. Edwards and H. V. Schaff, N. Engl. J. Med., 1997, 337, 581–588 CrossRef CAS PubMed.
- R. B. Rothman and M. H. Baumann, Am. J. Therapeut., 2009, 16, 354–364 CrossRef PubMed.
- M. H. Pittler and E. Ernst, Am. J. Med., 2001, 110, 724–730 CrossRef CAS.
- M. R. Stein, R. E. Julis, C. C. Peck, W. Hinshaw, J. E. Sawicki and J. J. Deller Jr., Am. J. Clin. Nutr., 1976, 9, 940–948 Search PubMed.
- E. P. Palmer and A. T. Guay, N. Engl. J. Med., 1985, 313, 1457–1459 CrossRef CAS PubMed.
- P. M. O’Neil, S. R. Smith, N. J. Weissman, M. C. Fidler, M. Sanchez, J. Zhang, B. Raether, C. M. Anderson and W. R. Shanahan, Obesity, 2012, 20, 1426–1436 CrossRef PubMed.
- B. Drew, A. Dixon and J. Dixon, Vasc. Health Risk Manage., 2007, 3, 817–821 CAS.
- D. Rucker, R. Padwal, S. K. Li, C. Curioni and D. C. Lau, BMJ, 2007, 335, 1194–1199 CrossRef CAS PubMed.
- J. H. Shin and K. M. Gadde, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2013, 6, 131–139 CAS.
- C. R. Lake, S. Gallant, E. Masson and P. Miller, Am. J. Med., 1990, 89, 195–208 CrossRef CAS.
- F. X. Pi-Sunyer, L. J. Aronne, H. M. Heshmati, J. Devin and J. Rosenstock, JAMA, 2006, 295, 761–775 CrossRef CAS PubMed.
- A. J. Scheen, J. Neuroendocrinol., 2008, 20, 139–146 CrossRef CAS PubMed.
- M. J. Perrio, L. V. Wilton and S. A. W. Shakir, Obesity, 2007, 15, 2712–2722 CrossRef PubMed.
- B. Hartung, M. Schott, T. Daldrup and S. Ritz-Timme, Int. J. Leg. Med., 2010, 124, 637–640 CrossRef PubMed.
- N. Yuda, M. Tanaka, M. Suzuki, Y. Asano, H. Ochi and K. Iwatsuki, J. Food Sci., 2012, 77, 254–261 CrossRef PubMed.
- M. Yamamoto, S. Shimura, Y. Itoh, T. Ohsaka, M. Egawa and S. Inoue, Int. J. Obes., 2000, 24, 758–764 CrossRef CAS PubMed.
- H. Sugiyama, Y. Akazome, T. Shoji, A. Yamaguchi, M. Yasue, T. Kanda and Y. Ohtake, J. Agric. Food Chem., 2007, 55, 4604–4609 CrossRef CAS PubMed.
- D. Moreno, N. Ilic, A. Poulev and I. Raskin, Life Sci., 2006, 78, 2797–2803 CrossRef CAS PubMed.
- S. Liu, D. Li, B. Huang, Y. Chen, X. Lu and Y. Wang, J. Ethnopharmacol., 2013, 26, 263–269 CrossRef PubMed.
- L. Anila and N. R. Vijayalakshmi, J. Ethnopharmacol., 2002, 79, 81–87 CrossRef CAS.
- M. Yoshikawa, H. Shimoda, N. Nishida, M. Takada and H. Matsuda, J. Nutr., 2002, 132, 1819–1824 CAS.
- Y. Guo, G. Wu, X. Su, H. Yang and J. Zhang, Nutr. Res., 2009, 29, 656–663 CrossRef CAS PubMed.
- K. Kawaguchi, T. Mizuno, K. Aida and K. Uchino, Biosci., Biotechnol., Biochem., 1997, 61, 102–104 CrossRef CAS PubMed.
- F. Martins, T. Noso, V. Porto, A. Curiel, A. Gambero and D. H. M. Bastos, Obesity, 2010, 18, 42–47 CrossRef CAS PubMed.
- J. Zhang, M. Kang, M. Kim, M. Kim, J. Song and Y. Lee, Nutr. Res. Pract., 2008, 2, 200–203 CrossRef PubMed.
- H. Quesada, J. M. del Bas, D. Pajuelo, S. Daz, J. Fernandez-Larrea and M. Pinent, Int. J. Obes., 2009, 33, 1007–1012 CrossRef CAS PubMed.
- H. Shimoda, E. Seki and M. Aitani, BMC Complementary Altern. Med., 2006, 6, 9 CrossRef PubMed.
- H. Kimura, S. Ogawa, T. Katsube, M. Jisaka and K. Yokota, J. Agric. Food Chem., 2008, 56, 4783–4788 CrossRef CAS PubMed.
- C. Kwon, H. Sohn, S. Kim, J. Kim, K. Son and J. Lee, Biosci., Biotechnol., Biochem., 2003, 67, 1451–1456 CrossRef CAS PubMed.
- K. Yoshizumi, K. Hirano, H. Ando, Y. Hirai, Y. Ida and T. Tsuji, J. Agric. Food Chem., 2006, 54, 335–341 CrossRef CAS PubMed.
- L. Sheng, Z. Qian, S. Zheng and L. Xi, Eur. J. Pharmacol., 2006, 543, 116–122 CrossRef CAS PubMed.
- Q. Zheng, W. Li, L. Han and K. Koike, Chem. Pharm. Bull., 2007, 55, 646–650 CrossRef CAS.
- N. Karu, R. Reifen and Z. Kerem, J. Agric. Food Chem., 2007, 55, 2824–2828 CrossRef CAS PubMed.
- L. K. Han, Y. N. Zheng, M. Yoshikawa, H. Okuda and Y. Kimura, BMC Complementary Altern. Med., 2005 DOI:10.1186/1472-6882-5-9.
- W. Liu, Y. Zheng, L. Han, H. Wang, M. Saito and M. Ling, Phytomedicine, 2008, 15, 1140–1145 CrossRef CAS PubMed.
- T. Morikawa, Y. Xie, Y. Asao, M. Okamoto, C. Yamashita and O. Muraoka, Phytochemistry, 2009, 70, 1166–1172 CrossRef CAS PubMed.
- L. K. Han, Y. Kimura, M. Kawashima, T. Takaku, T. Taniyama and T. Hayashi, Int. J. Obes., 2001, 25, 1459–1464 CrossRef CAS PubMed.
- S. Jäger, H. Trojan, T. Kopp, M. Laszczyk and A. Scheffler, Molecules, 2009, 14, 2016–2031 CrossRef PubMed.
- P. Slanc, B. Doljak, S. Kreft, M. Lunder, D. Janes and B. Strukelj, Phytother. Res., 2009, 23, 874–877 CrossRef CAS PubMed.
- M. Mutoh, N. Nakada, S. Matsukuma, S. Ohshima, K. Yoshinari and J. Watanabe, J. Antibiot., 1994, 47, 1369–1375 CrossRef CAS.
- M. Kitahara, M. Asano, H. Naganawa, K. Maeda, M. Hamada, T. Aoyagi, H. Umezawa, Y. Iitaka and H. Nakamura, J. Antibiot., 1987, 40, 1647–1650 CrossRef CAS.
- H. Umezawa, T. Aoyagi, T. Hazato, K. Uotani, F. Kojima, M. Hamada and T. Takeuchi, J. Antibiot., 1978, 31, 639–641 CrossRef CAS.
- T. Imanaka, Y. Moriyama, G. G. Ecsedi, T. Aoyagi, K. Amanuma-Muto, S. Ohkuma and T. Takano, J. Biochem., 1983, 94, 1017–1020 CAS.
- N. Bitou, M. Ninomiyaa, T. Tsujitab and H. Okuda, Lipids, 1999, 34, 441–445 CrossRef CAS.
- M. Matsumoto, M. Hosokawa, N. Matsukawa, M. Hagio, A. Shinoki, M. Nishimukai, K. Miyashita, T. Yajima and H. Hara, Eur. J. Nutr., 2010, 49, 243–249 CrossRef CAS PubMed.
- J. Lee, J. Song and J. Lee, Mycobiology, 2010, 38, 58–61 CrossRef CAS PubMed.
- D. Z. Liu, F. Wang, T. G. Liao, J. G. Tang, W. Steglich, H. J. Zhu and J. K. Liu, Org. Lett., 2006, 8, 5749–5752 CrossRef CAS PubMed.
- J. Kim, H. Kim, H. Park, S. Youn, D. Choi and C. Shin, FEMS Microbiol. Lett., 2007, 276, 93–98 CrossRef CAS PubMed.
- H. Cordula, K. Michael, M. Guenter and T. Luigi, http://patentscope.wipo.int/search/en/WO2001077094, 2001.
| This journal is © The Royal Society of Chemistry 2015 |