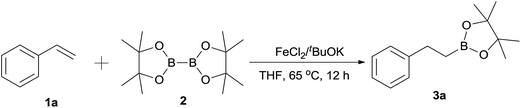
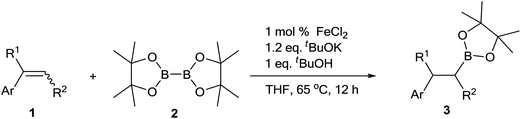
FeCl2-catalyzed hydroboration of aryl alkenes with bis(pinacolato)diboron†
Yang Liu,
Yuhan Zhou*,
Huan Wang and
Jingping Qu
State Key Laboratory of Fine Chemicals, School of Pharmaceutical Science and Technology, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: zhouyh@dl.cn
First published on 24th August 2015
Abstract
The first ligand-free ferrous chloride catalyzed anti-Markovnikov hydroboration of un-activated aryl alkenes with bis(pinacolato)diboron (B2pin2) has been reported. The reactions proceeded smoothly with high regioselectivity for a large range of aryl alkenes with wide functional-group compatibility and low catalyst loading under mild conditions.
Introduction
Alkylboronates are among the most versatile building blocks in organic synthesis. They are not only widely used in Suzuki–Miyaura cross-coupling reactions with aryl and alkyl halides but can also be readily converted into the corresponding alcohols, aldehydes, amines, etc.1 An advantage of alkylboronates over other common C(sp3) organometallic nucleophiles like Grignard reagent is their stability. Many of them can be stored in air and readily purified as common organic compounds by chromatography.1d For this reason, the synthesis of alkylboronates with diverse functional group is especially meaningful.Traditionally, alkylboronates are prepared by the reactions of suitable boron reagents with Grignard reagent or organolithium reagents.1a,2 However, these methods are limited due to poor functional-group compatibility and atom economy. Recently, transition metal-catalyzed borylations of alkanes,3 alkenes,4–8 alkyl halides and pseudohalides,9 as an alternative method for the preparation of alkylboronates, have become one of the most active research topics. Among them, transition metal-catalyzed hydroboration of alkenes is a useful method for the preparation of alkylboronates, owing to wide functional-group compatibility and mild conditions. Significant progress has been made recently in such transformations catalyzed by rhodium,5 iridium,5b,5f,5i,6 copper,7 cobalt,8 etc.
In consideration of sustainable and green chemistry, iron-based catalytic systems have received significant growing interests because iron salts are earth-abundant, inexpensive, environment-friendly and less toxic.10 Beyond the traditional iron-catalyzed reactions, such as Friedel–Crafts reaction, oxidation, etc., the good performance of iron-catalysts on hydrosilylation,11 hydrogenation,12 cross-coupling,13 C–H activation,14 cyclization,15 addition of unsaturated C–C bonds16 has made it a rising star in catalyst. However, there are only several examples of iron-catalyzed borylation reactions of alkenes. Ritter and co-workers17 discovered the first iron-catalyzed hydroboration of 1,3-dienes to afford linear (E)-γ-disubstituted allylboranes with pinacolborane (HBpin). In recent years, Huang,18 Thomas,19 Chirik,20 Lu,21 Szymczak,22 Darcel23 and co-workers successively reported iron-catalyzed hydroboration of alkenes with HBpin. However, most of these synthetic methods need adding reductant, like NaBHEt3 or Grignard reagent. In addition, the use of ligands or complicated iron complex increased their cost in application. Fernández and co-workers24 reported a commercially available iron salts catalyzed hydroboration of electron-deficient olefins with B2pin2. However, the substrates are limited to α,β-unsaturated esters, ketones, and imines. Thus, the commercially available iron salt catalyzed hydroboration of simple alkenes with B2pin2 is still highly desirable. In this context, we report a ferrous chloride catalyzed hydroboration of aryl alkenes with B2pin2 in the absence of ligands.
Results and discussion
Our studies were initiated by choosing hydroboration of styrene (1a) with B2pin2 (2) as the model reaction (Table 1). Fortunately, the first catalytic reaction performed with FeCl2 as catalyst upon addition of tBuOK and tBuOH in THF stirring at 65 °C for 12 h afforded 4,4,5,5-tetramethyl-2-phenethyl-1,3,2-dioxaborolane (3a) in 100% yield (Table 1, entry 1) with high regioselectivity. Three control experiments were performed to ensure this reaction was catalyzed by iron salt (Table 1, entries 2–4). Using high-purity (99.99%) FeCl2 as catalyst can also afford a quantitative yield (Table 1, entry 2). When FeCl3 was used, a slightly declining yield was observed in 90% (Table 1, entry 3). It is not surprising that there was almost no reaction in absence of iron salt (Table 1, entry 4). So, this reaction catalyzed by iron salt was identified. At lower temperature, the reaction can also proceed but was significantly slow. Only 24% yield was achieved at room temperature even for 24 h (Table 1, entry 5).| Entry | Catalyst | tBuOK | B2pin2 | Additive | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: experiments were performed with 1a (0.4 mmol), 2, FeCl2, tBuOK, additive (0.4 mmol, 1 eq.) in THF (5 mL) at 65 °C for 12 h.b Yield are determined by 1H NMR with 2,2,2-trichloroethyl 2,2,2-trichloroacetate as an internal standard.c High-purity (99.99%) FeCl2 was used.d Reaction was performed at 25 °C for 24 h.e Isolated yield.f 1a (2 mmol), THF (20 mL).g 1a (20 mmol), THF (100 mL). | |||||
| 1 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | tBuOH | 100 |
| 2 | FeCl2 (10 mol%)c | 1.2 eq. | 1.5 eq. | tBuOH | 100 |
| 3 | FeCl3 (10 mol%) | 1.2 eq. | 1.5 eq. | tBuOH | 90 |
| 4 | — | 1.2 eq. | 1.5 eq. | tBuOH | Trace |
| 5 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | tBuOH | 24d |
| 6 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | MeOH | 96 |
| 7 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | EtOH | 97 |
| 8 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | iPrOH | 97 |
| 9 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | H2O | 64 |
| 10 | FeCl2 (10 mol%) | 1.2 eq. | 1.5 eq. | — | 10 |
| 11 | FeCl2 (10 mol%) | — | 1.5 eq. | tBuOH | Trace |
| 12 | FeCl2 (10 mol%) | 1.2 eq. | 0.6 eq. | tBuOH | 42 |
| 13 | FeCl2 (10 mol%) | 1.2 eq. | 1 eq. | tBuOH | 76 |
| 14 | FeCl2 (10 mol%) | 0.6 eq. | 1.5 eq. | tBuOH | 82 |
| 15 | FeCl2 (10 mol%) | 1 eq. | 1.5 eq. | tBuOH | 93 |
| 16 | FeCl2 (1 mol%) | 1.2 eq. | 1.5 eq. | tBuOH | 92e,f |
| 17 | FeCl2 (0.1 mol%) | 1.2 eq. | 1.5 eq. | tBuOH | 59e,g |
| 18 | FeCl2 (1 mol%)c | 1.2 eq. | 1.5 eq. | tBuOH | 90e,f |
The fact that the addition of alcohols or water is essential in the hydroboration of alkenes has been confirmed by previous reports.7e Consequently, the effect of alcohols or water was investigated. If no additional alcohols or water was added, the yield of 3a was very low (Table 1, entry 10). When 1 equivalent of alcohols or water was added, the yield of 3a increased sharply (Table 1, entry 1 and entries 6–9). In general, alcohols were better than water, and the best result was achieved by tBuOH.
In addition, the base is also essential for this reaction. There was almost no reaction in absence of tBuOK (Table 1, entry 11). Further study showed that a slight excess tBuOK and B2pin2 were beneficial for the conversion (Table 1, entry 1 and entries 12–15), and the best result was obtained with 1.2 equivalent of tBuOK and 1.5 equivalent of B2pin2 (Table 1, entry 1). The catalytic activity of FeCl2 was so high that an excellent isolated yield of 92% was achieved even when the amount of catalyst was reduced to 1 mol% (Table 1, entry 16), and using high-purity (99.99%) FeCl2 as catalyst can also afford a high isolated yield (Table 1, entry 18). But further reducing of the catalyst resulted in the sharp decrease in yield (Table 1, entry 17).
Solvents play an important role in affecting reactivity (Table 2). Good yield was obtained with THF but not for dioxane and tBuOMe (Table 2, entries 1–3). Alcohol solvents show a tremendous difference in yield (Table 2, entries 4 and 5). Nonpolar solvents such as CCl4 and toluene were not suitable for the reaction (Table 2, entries 6 and 7). Polar solvents like MeCN, DMSO and DMF led to a moderate to excellent yield (Table 2, entries 8–10).
| Entry | Solvent | Yieldb (%) |
|---|---|---|
| a Conditions: experiments were performed with 1a (0.4 mmol), 2 (0.6 mmol), FeCl2 (0.04 mmol), tBuOK (0.48 mmol), tBuOH (0.4 mmol) in solvent (5 mL) at 65 °C for 12 h.b Yield are determined by 1H NMR with 2,2,2-trichloroethyl 2,2,2-trichloroacetate as an internal standard. | ||
| 1 | THF | 100 |
| 2 | Dioxane | 24 |
| 3 | tBuOMe | 43 |
| 4 | tBuOH | 95 |
| 5 | MeOH | 43 |
| 6 | CCl4 | 0 |
| 7 | Toluene | 50 |
| 8 | MeCN | 77 |
| 9 | DMSO | 70 |
| 10 | DMF | 95 |
The scope of this reaction was explored as the optimized conditions identified. A range of substituted aryl alkenes were initially surveyed (Table 3). Both electron-donating and electron-withdrawing groups were tolerated in this reaction. A variety of para-substituted functional groups, such as methyl, methoxyl, cyano, amine, ester and halogen were compatible to generate desired alkylboronates (3b–k) in good to excellent yields. Besides, a chloro substituent was tolerated at all the positions on the benzene ring (3h, 3j and 3k) without any dehalogenated byproducts. However, reaction of 4-bromostyrene (1i) as substrate under the optimized condition gave both the desired product (3i) and dehalogenation byproduct (3a). In order to get higher yield, the reaction time was decreased to 3 h, and the desired product was afforded in 80% yield. The polysubstituted styrene, thienyl alkene, furyl alkene and naphthyl alkenes also proceeded smoothly with 2 to afford moderate to good yields of product independently. In addition, 1,3-divinylbenzene could be employed as substrate to give outstanding yield of the corresponding product (3o) with diploid loading of other reagents and catalyst but not reaction time. To our delight, the hydroboration of 1,1-disubstituted alkenes also took place smoothly to give the product (3p–r) in excellent yield. Furthermore, internal alkenes, including furyl alkene, phenyl alkene and naphthyl alkene provided the desired product in moderate to good yields (3s–v). Finally, another series of cyclic aryl alkenes, such as indene and 1,2-dihydronaphthalene, can also undergo reaction to obtain desired products in high yield (3w and 3x). Unfortunately, under the optimized condition, the reaction almost no occurred when using cyclohexene as a substrate. Using 1-octene as a substrate, the product was afforded in low isolated yield of 31%, with a low regioselectivity of 30% of the branched isomer and 70% of the linear isomer.
| a Conditions: experiments were performed with 1 (2 mmol), 2 (3 mmol), FeCl2 (0.02 mmol), tBuOK (2.4 mmol), tBuOH (2 mmol) in THF (20 mL) at 65 °C for 12 h.b Isolated yields.c Reaction was performed for 3 h.d Reaction was performed with 1o (2 mmol), 2 (6 mmol), FeCl2 (0.04 mmol), tBuOK (4.8 mmol), tBuOH (4 mmol) in THF (30 mL) at 65 °C for 12 h. |
|---|
 |
On the basis of the above information and previous reports,7l,17,18a,25 a putative reaction pathway for the iron-catalyzed hydroboration of aryl alkenes with B2pin2 is shown in Scheme 1. First, complex A was generated via addition of tBuOK to B2pin2. Then, upon the cleavage of B–B bond in complex A, the addition of −Bpin anion to the iron center afford intermediate B. Species C was formed by coordination of aryl alkene to the intermediate B followed by the insertion of aryl alkene to the Fe–B bond. Finally, intermediate C was protonated with tBuOH to generate anti-Markovnikov products and the iron catalyst. In order to verify the proton supplied with additive, isotope labeling experiment was performed under the standard reaction conditions but the tBuOH was replaced by CD3OD (Scheme 2). The deuterated product (3a-D) was isolated with a good yield of 85% and high deuterated ratio in 99%. tBuOK did not consume in our proposed catalytic cycle, but 1.2 equivalent of tBuOK was needed for high yield of the product in the best condition. Our simulative argument is that a high concentration of intermediate B is essential to generate species C. And the result also showed that a yield of 82% was achieved when 0.6 equivalent of tBuOK was used (Table 1, entry 14). That means the stoichiometric tBuOK is not essential for the reaction. It agrees with our proposed mechanism.
Experimental section
Materials and methods
Unless otherwise noted, all reactions were performed under argon using Schlenk line techniques with magnetic stirring. Solvents were dried by passage through an activated alumina column under argon. Column chromatography was performed on silica gel (200–300 mesh) or aluminum oxide (neutral, 200–300 mesh). All 1H NMR (400 MHz) and 13C NMR (100 MHz) or 13C NMR (125 MHz) were recorded on Bruker AVANCE II-400 or Bruker AVANCE III-500 spectrometer with chemical shifts reported as ppm (in CDCl3, with TMS as an internal standard). High resolution mass spectra (HRMS) (EI) were recorded on a Micromass GCT spectrometer.Aryl alkenes 1e, 1l, 1o, 1s (mixture of cis- and trans isomers), 1t (mixture of cis- and trans isomers), 1u (mixture of cis- and trans isomers) and 1v (mixture of cis- and trans isomers) were prepared by Wittig olefination of the corresponding aldehyde followed the examples of previously reported procedures.26 Other aryl alkenes were purchased and used as received.
The following chemicals were purchased and used as received: FeCl3 (98%, J&K), tBuOK (99%, J&K), bis(pinacolato)diboron (>99%, TCI), high-purity FeCl2 (beads, 99.99%, Sigma-Aldrich, powder was prepared using a mortar and pestle in a glovebox).
FeCl2 was prepared from FeCl3 (50 g) in chlorobenzene (450 mL) at 130 °C for 8 h under argon followed the example of previously reported procedures.27
General procedure for the synthesis of alkylboronates
To a Schlenk tube equipped with a magnetic stir bar and charged with FeCl2 (2.6 mg, 0.02 mmol) was added THF (20 mL), followed by aryl alkene (2 mmol), bis(pinacolato)diboron (762 mg, 3 mmol), tBuOK (269 mg, 2.4 mmol), tBuOH (150 mg, 2 mmol). The resulting solution was stirred at 65 °C for 12 h. The solution of the crude product was concentrated in vacuum, brine (20 mL) was added and the aqueous layer was extracted with EtOAc (3 × 20 mL). The combined organic layers were dried (Na2SO4) and the solvent was removed under reduced pressure. The resultant crude product material was purified by flash chromatography using the appropriate gradient of petroleum ether and EtOAc.4,4,5,5-Tetramethyl-2-phenethyl-1,3,2-dioxaborolane (3a)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (430 mg, 92%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.20–7.27 (m, 4H, ArH), 7.13–7.16 (m, 1H, ArH), 2.75 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.14 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 144.5, 128.3, 128.1, 125.6, 83.2, 30.1, 24.7. These spectroscopic data correspond to reported data.7m
3). Colorless oil (430 mg, 92%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.20–7.27 (m, 4H, ArH), 7.13–7.16 (m, 1H, ArH), 2.75 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.14 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 144.5, 128.3, 128.1, 125.6, 83.2, 30.1, 24.7. These spectroscopic data correspond to reported data.7m
4,4,5,5-Tetramethyl-2-(4-methylphenethyl)-1,3,2-dioxaborolane (3b)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (421 mg, 86%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.04–7.12 (m, 4H, ArH), 2.70 (t, J = 8.0 Hz, 2H, ArCH2), 2.30 (s, 3H, ArCH3), 1.23 (s, 12H, 2C(CH3)2), 1.12 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 141.2, 134.6, 128.8, 127.8, 82.8, 29.5, 24.7, 20.9. These spectroscopic data correspond to reported data.7m
3). Colorless oil (421 mg, 86%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.04–7.12 (m, 4H, ArH), 2.70 (t, J = 8.0 Hz, 2H, ArCH2), 2.30 (s, 3H, ArCH3), 1.23 (s, 12H, 2C(CH3)2), 1.12 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 141.2, 134.6, 128.8, 127.8, 82.8, 29.5, 24.7, 20.9. These spectroscopic data correspond to reported data.7m
2-(4-Methoxyphenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3c)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Colorless oil (464 mg, 89%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.13 (d, J = 8.0 Hz, 2H, ArH), 6.81 (d, J = 8.0 Hz, 2H, ArH), 3.78 (s, 3H, OCH3), 2.69 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 157.6, 136.5, 128.8, 113.6, 83.0, 55.2, 29.1, 24.8. These spectroscopic data correspond to reported data.7m
5). Colorless oil (464 mg, 89%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.13 (d, J = 8.0 Hz, 2H, ArH), 6.81 (d, J = 8.0 Hz, 2H, ArH), 3.78 (s, 3H, OCH3), 2.69 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 157.6, 136.5, 128.8, 113.6, 83.0, 55.2, 29.1, 24.8. These spectroscopic data correspond to reported data.7m
2-(4-Cyanophenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3d)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Colorless oil (497 mg, 97%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.55 (d, J = 8.0 Hz, 2H, ArH), 7.31 (d, J = 8.0 Hz, 2H, ArH), 2.79 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.14 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 150.1, 132.0, 128.9, 119.2, 109.4, 83.3, 30.1, 24.8. These spectroscopic data correspond to reported data.28
7). Colorless oil (497 mg, 97%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.55 (d, J = 8.0 Hz, 2H, ArH), 7.31 (d, J = 8.0 Hz, 2H, ArH), 2.79 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.14 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 150.1, 132.0, 128.9, 119.2, 109.4, 83.3, 30.1, 24.8. These spectroscopic data correspond to reported data.28
4,4,5,5-Tetramethyl-2-(4-dimethylaminophenethyl)-1,3,2-dioxaborolane (3e)
Title compound was isolated by flash chromatography on aluminum oxide eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Pale yellow oil (460 mg, 84%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.10 (d, J = 8.0 Hz, 2H, ArH), 6.69 (d, J = 8.0 Hz, 2H, ArH), 2.90 (s, 6H, N(CH3)2), 2.66 (t, J = 8.0 Hz, 2H, ArCH2), 1.23 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 149.0, 133.0, 128.5, 113.1, 83.1, 41.1, 29.0, 24.9; HRMS-EI: calc. for C16H26O2BN, 275.2057; found, 275.2061.
7). Pale yellow oil (460 mg, 84%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.10 (d, J = 8.0 Hz, 2H, ArH), 6.69 (d, J = 8.0 Hz, 2H, ArH), 2.90 (s, 6H, N(CH3)2), 2.66 (t, J = 8.0 Hz, 2H, ArCH2), 1.23 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 149.0, 133.0, 128.5, 113.1, 83.1, 41.1, 29.0, 24.9; HRMS-EI: calc. for C16H26O2BN, 275.2057; found, 275.2061.
2-(4-Methoxycarbonylphenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3f)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). Colorless oil (396 mg, 68%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.93 (d, J = 8.0 Hz, 2H, ArH), 7.28 (d, J = 8.0 Hz, 2H, ArH), 3.89 (s, 3H, OCH3), 2.79 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.15 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 167.1, 149.9, 129.6, 128.0, 127.6, 83.2, 51.8, 30.0, 24.8. These spectroscopic data correspond to reported data.28
15). Colorless oil (396 mg, 68%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.93 (d, J = 8.0 Hz, 2H, ArH), 7.28 (d, J = 8.0 Hz, 2H, ArH), 3.89 (s, 3H, OCH3), 2.79 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.15 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 167.1, 149.9, 129.6, 128.0, 127.6, 83.2, 51.8, 30.0, 24.8. These spectroscopic data correspond to reported data.28
2-(4-Fluorophenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3g)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (495 mg, 99%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.13–7.18 (m, 2H, ArH), 6.90–6.96 (m, 2H, ArH), 2.71 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 161.2 (d, J = 241.6 Hz), 140.0 (d, J = 3.1 Hz), 129.4 (d, J = 7.6 Hz), 114.8 (d, J = 20.9 Hz), 83.1, 29.2, 24.8. These spectroscopic data correspond to reported data.7m
3). Colorless oil (495 mg, 99%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.13–7.18 (m, 2H, ArH), 6.90–6.96 (m, 2H, ArH), 2.71 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 161.2 (d, J = 241.6 Hz), 140.0 (d, J = 3.1 Hz), 129.4 (d, J = 7.6 Hz), 114.8 (d, J = 20.9 Hz), 83.1, 29.2, 24.8. These spectroscopic data correspond to reported data.7m
2-(4-Chlorophenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3h)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (523 mg, 98%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.22 (d, J = 8.0 Hz, 2H, ArH), 7.14 (d, J = 8.0 Hz, 2H, ArH), 2.71 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 142.8, 131.2, 129.4, 128.2, 83.2, 29.3, 24.8. These spectroscopic data correspond to reported data.7m
3). Colorless oil (523 mg, 98%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.22 (d, J = 8.0 Hz, 2H, ArH), 7.14 (d, J = 8.0 Hz, 2H, ArH), 2.71 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 142.8, 131.2, 129.4, 128.2, 83.2, 29.3, 24.8. These spectroscopic data correspond to reported data.7m
2-(4-Bromophenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3i)
Prepared by general procedure, but the reaction time was reduced to 3 h. Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (496 mg, 80%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.37 (d, J = 8.0 Hz, 2H, ArH), 7.08 (d, J = 8.0 Hz, 2H, ArH), 2.69 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 143.5, 131.3, 129.9, 119.3, 83.3, 29.5, 24.9. These spectroscopic data correspond to reported data.7m
3). Colorless oil (496 mg, 80%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.37 (d, J = 8.0 Hz, 2H, ArH), 7.08 (d, J = 8.0 Hz, 2H, ArH), 2.69 (t, J = 8.0 Hz, 2H, ArCH2), 1.21 (s, 12H, 2C(CH3)2), 1.11 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 143.5, 131.3, 129.9, 119.3, 83.3, 29.5, 24.9. These spectroscopic data correspond to reported data.7m
2-(2-Chlorophenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3j)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (520 mg, 98%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.25–7.32 (m, 2H, ArH), 7.08–7.18 (m, 2H, ArH), 2.84 (t, J = 8.0 Hz, 2H, ArCH2), 1.24 (s, 12H, 2C(CH3)2), 1.15 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 141.9, 133.9, 129.8, 129.4, 127.1, 126.7, 83.2, 27.9, 24.9; HRMS-EI: calc. for C14H20O2BCl, 266.1245; found, 266.1256.
3). Colorless oil (520 mg, 98%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.25–7.32 (m, 2H, ArH), 7.08–7.18 (m, 2H, ArH), 2.84 (t, J = 8.0 Hz, 2H, ArCH2), 1.24 (s, 12H, 2C(CH3)2), 1.15 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 141.9, 133.9, 129.8, 129.4, 127.1, 126.7, 83.2, 27.9, 24.9; HRMS-EI: calc. for C14H20O2BCl, 266.1245; found, 266.1256.
2-(3-Chlorophenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3k)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (520 mg, 98%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.07–7.24 (m, 4H, ArH), 2.72 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.12 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 146.4, 133.9, 129.4, 128.3, 126.3, 125.7, 83.2, 29.7, 24.8; HRMS-EI: calc. for C14H20O2BCl, 266.1245; found, 266.1238.
3). Colorless oil (520 mg, 98%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.07–7.24 (m, 4H, ArH), 2.72 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.12 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 146.4, 133.9, 129.4, 128.3, 126.3, 125.7, 83.2, 29.7, 24.8; HRMS-EI: calc. for C14H20O2BCl, 266.1245; found, 266.1238.
2-(3,4,5-Trimethoxyphenethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3l)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). White solid (622 mg, 97%); m.p. 61–63 °C; 1H NMR (400 MHz, CDCl3, Me4Si) δ 6.44 (s, 2H, ArH), 3.83 (s, 6H, 2OCH3), 3.81 (s, 3H, OCH3), 2.69 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.14 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 153.0, 140.3, 135.9, 104.9, 83.2, 60.9, 56.0, 30.4, 24.9; HRMS-EI: calc. for C17H27O5B, 322.1952; found, 322.1959.
7). White solid (622 mg, 97%); m.p. 61–63 °C; 1H NMR (400 MHz, CDCl3, Me4Si) δ 6.44 (s, 2H, ArH), 3.83 (s, 6H, 2OCH3), 3.81 (s, 3H, OCH3), 2.69 (t, J = 8.0 Hz, 2H, ArCH2), 1.22 (s, 12H, 2C(CH3)2), 1.14 (t, J = 8.0 Hz, 2H, BCH2); 13C NMR (100 MHz, CDCl3) δ 153.0, 140.3, 135.9, 104.9, 83.2, 60.9, 56.0, 30.4, 24.9; HRMS-EI: calc. for C17H27O5B, 322.1952; found, 322.1959.
2-(2-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)ethyl)-thiophene (3m)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Pale yellow oil (315 mg, 66%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.07–7.09 (m, 1H, ArH), 6.88–6.90 (m, 1H, ArH), 6.79–6.80 (m, 1H, ArH), 2.96 (t, J = 8.0 Hz, 2H, ArCH2), 1.24–1.20 (m, 14H, 2C(CH3)2 + BCH2); 13C NMR (125 MHz, CDCl3) δ 147.7, 126.5, 123.4, 122.6, 83.2, 24.8, 24.4. These spectroscopic data correspond to reported data.9a
3). Pale yellow oil (315 mg, 66%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.07–7.09 (m, 1H, ArH), 6.88–6.90 (m, 1H, ArH), 6.79–6.80 (m, 1H, ArH), 2.96 (t, J = 8.0 Hz, 2H, ArCH2), 1.24–1.20 (m, 14H, 2C(CH3)2 + BCH2); 13C NMR (125 MHz, CDCl3) δ 147.7, 126.5, 123.4, 122.6, 83.2, 24.8, 24.4. These spectroscopic data correspond to reported data.9a
4,4,5,5-Tetramethyl-2-(2-(naphthalen-1-yl)ethyl)-1,3,2-dioxaborolane (3n)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (500 mg, 89%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 8.09 (d, J = 8.0 Hz, 1H, ArH), 7.84 (d, J = 8.0 Hz, 1H, ArH), 7.65–7.71 (m, 1H, ArH), 7.44–7.52 (m, 2H, ArH), 7.36–7.41 (m, 2H, ArH), 3.21 (t, J = 8.0 Hz, 2H, ArCH2), 1.29 (t, J = 8.0 Hz, 2H, BCH2), 1.25 (s, 12H, 2C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 140.5, 133.9, 131.8, 128.7, 126.4, 125.7, 125.6, 125.4, 125.1, 124.0, 83.2, 27.0, 24.9; HRMS-EI: calc. for C18H23O2B, 282.1791; found, 282.1790.
3). Colorless oil (500 mg, 89%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 8.09 (d, J = 8.0 Hz, 1H, ArH), 7.84 (d, J = 8.0 Hz, 1H, ArH), 7.65–7.71 (m, 1H, ArH), 7.44–7.52 (m, 2H, ArH), 7.36–7.41 (m, 2H, ArH), 3.21 (t, J = 8.0 Hz, 2H, ArCH2), 1.29 (t, J = 8.0 Hz, 2H, BCH2), 1.25 (s, 12H, 2C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 140.5, 133.9, 131.8, 128.7, 126.4, 125.7, 125.6, 125.4, 125.1, 124.0, 83.2, 27.0, 24.9; HRMS-EI: calc. for C18H23O2B, 282.1791; found, 282.1790.
1,3-Bis(2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)ethyl)benzene (3o)
To a Schlenk tube equipped with a magnetic stir bar and charged with FeCl2 (5.1 mg, 0.04 mmol) was added THF (20 mL), followed by 1,3-divinylbenzene (2 mmol), bis(pinacolato)diboron (1524 mg, 6 mmol), tBuOK (538 mg, 4.8 mmol), tBuOH (300 mg, 4 mmol). The resulting solution was stirred at 65 °C for 12 h. The solution of the crude product was concentrated in vacuo, brine (20 mL) was added and the aqueous layer was extracted with EtOAc (3 × 20 mL). The combined organic layers were dried (Na2SO4) and the solvent removed under reduced pressure. Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). White solid (740 mg, 96%); m.p. 103–105 °C; 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.15 (t, J = 8.0 Hz, 1H, ArH), 7.06 (s, 1H, ArH), 7.01 (d, J = 8.0 Hz, 2H, 2ArH), 2.70 (t, J = 8.0 Hz, 4H, 2PhCH2), 1.22 (s, 24H, 4C(CH3)2), 1.12 (t, J = 8.0 Hz, 4H, 2BCH2); 13C NMR (100 MHz, CDCl3) δ 144.2, 128.0, 127.8, 125.2, 83.0, 29.9, 24.8; HRMS-EI: calc. for C22H36O4B2, 386.2800; found, 386.2806.
7). White solid (740 mg, 96%); m.p. 103–105 °C; 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.15 (t, J = 8.0 Hz, 1H, ArH), 7.06 (s, 1H, ArH), 7.01 (d, J = 8.0 Hz, 2H, 2ArH), 2.70 (t, J = 8.0 Hz, 4H, 2PhCH2), 1.22 (s, 24H, 4C(CH3)2), 1.12 (t, J = 8.0 Hz, 4H, 2BCH2); 13C NMR (100 MHz, CDCl3) δ 144.2, 128.0, 127.8, 125.2, 83.0, 29.9, 24.8; HRMS-EI: calc. for C22H36O4B2, 386.2800; found, 386.2806.
4,4,5,5-Tetramethyl-2-(2-phenylpropyl)-1,3,2-dioxaborolane (3p)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (490 mg, 99%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.22–7.28 (m, 4H, ArH), 7.13–7.16 (m, 1H, ArH), 2.98–3.07 (m, 1H, ArCH), 1.27 (d, 3H, CHCH3), 1.14–1.16 (m, 14H, 2C(CH3)2 + BCH2); 13C NMR (125 MHz, CDCl3) δ 149.2, 128.2, 126.6, 125.7, 82.9, 35.8, 25.0, 24.8, 24.7. These spectroscopic data correspond to reported data.7m
3). Colorless oil (490 mg, 99%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.22–7.28 (m, 4H, ArH), 7.13–7.16 (m, 1H, ArH), 2.98–3.07 (m, 1H, ArCH), 1.27 (d, 3H, CHCH3), 1.14–1.16 (m, 14H, 2C(CH3)2 + BCH2); 13C NMR (125 MHz, CDCl3) δ 149.2, 128.2, 126.6, 125.7, 82.9, 35.8, 25.0, 24.8, 24.7. These spectroscopic data correspond to reported data.7m
2-(2,2-Diphenylethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3q)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Pale yellow solid (577 mg, 94%); m.p. 60–62 °C; 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.19–7.28 (m, 8H, ArH), 7.11–7.15 (m, 2H, ArH), 4.28 (t, J = 8.0 Hz, 1H, ArCH), 1.60 (d, J = 8.0 Hz, 2H, BCH2), 1.05 (s, 12H, 2C(CH3)2); 13C NMR (125 MHz, CDCl3) δ 146.7, 128.3, 127.8, 126.0, 83.2, 46.7, 24.7. These spectroscopic data correspond to reported data.7m
3). Pale yellow solid (577 mg, 94%); m.p. 60–62 °C; 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.19–7.28 (m, 8H, ArH), 7.11–7.15 (m, 2H, ArH), 4.28 (t, J = 8.0 Hz, 1H, ArCH), 1.60 (d, J = 8.0 Hz, 2H, BCH2), 1.05 (s, 12H, 2C(CH3)2); 13C NMR (125 MHz, CDCl3) δ 146.7, 128.3, 127.8, 126.0, 83.2, 46.7, 24.7. These spectroscopic data correspond to reported data.7m
4,4,5,5-Tetramethyl-2-(2-(naphthalen-2-yl)propyl)-1,3,2-dioxaborolane (3r)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (555 mg, 94%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.78–7.82 (m, 3H, ArH), 7.69 (s, 1H, ArH) 7.40–7.48 (m, 3H, ArH), 2.21–3.30 (m, 1H, ArCH), 1.40 (d, J = 4.0 Hz, 3H, CHCH3), 1.29 (t, J = 8.0 Hz, 2H, BCH2), 1.17 (s, 12H, 2C(CH3)2); 13C NMR (125 MHz, CDCl3) δ 146.8, 133.7, 132.2, 127.8, 127.7, 127.6, 126.0, 125.8, 125.0, 124.5, 83.1, 36.0, 24.9, 24.8, 24.8. These spectroscopic data correspond to reported data.7i
3). Colorless oil (555 mg, 94%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.78–7.82 (m, 3H, ArH), 7.69 (s, 1H, ArH) 7.40–7.48 (m, 3H, ArH), 2.21–3.30 (m, 1H, ArCH), 1.40 (d, J = 4.0 Hz, 3H, CHCH3), 1.29 (t, J = 8.0 Hz, 2H, BCH2), 1.17 (s, 12H, 2C(CH3)2); 13C NMR (125 MHz, CDCl3) δ 146.8, 133.7, 132.2, 127.8, 127.7, 127.6, 126.0, 125.8, 125.0, 124.5, 83.1, 36.0, 24.9, 24.8, 24.8. These spectroscopic data correspond to reported data.7i
2-(1-(Furan-2-yl)pentan-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3s)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (337 mg, 64%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.26 (d, J = 4.0 Hz, 1H, ArH), 6.24 (t, J = 4.0 Hz, 1H, ArH), 5.97 (d, J = 4.0 Hz, 1H, ArH), 2.74 (dd, J = 8.0 Hz, J = 15.00 Hz, 1H, 1/2ArCH2), 2.66 (dd, J = 8.0 Hz, J = 15.00 Hz, 1H, 1/2ArCH2), 1.28–1.48 (m, 5H, BCH + CHCH2 + CH3CH2), 1.20 (s, 6H, 2 × [1/2C(CH3)2]), 1.12 (s, 6H, 2 × [1/2C(CH3)2]), 0.89 (t, J = 8.0 Hz, 3H, CH2CH3); 13C NMR (100 MHz, CDCl3) δ 156.3, 140.6, 110.0, 105.1, 83.1, 33.4, 29.6, 24.8, 24.8, 22.2, 14.4; HRMS-EI: calc. for C15H25O3B, 264.1897; found, 264.1899.
3). Colorless oil (337 mg, 64%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.26 (d, J = 4.0 Hz, 1H, ArH), 6.24 (t, J = 4.0 Hz, 1H, ArH), 5.97 (d, J = 4.0 Hz, 1H, ArH), 2.74 (dd, J = 8.0 Hz, J = 15.00 Hz, 1H, 1/2ArCH2), 2.66 (dd, J = 8.0 Hz, J = 15.00 Hz, 1H, 1/2ArCH2), 1.28–1.48 (m, 5H, BCH + CHCH2 + CH3CH2), 1.20 (s, 6H, 2 × [1/2C(CH3)2]), 1.12 (s, 6H, 2 × [1/2C(CH3)2]), 0.89 (t, J = 8.0 Hz, 3H, CH2CH3); 13C NMR (100 MHz, CDCl3) δ 156.3, 140.6, 110.0, 105.1, 83.1, 33.4, 29.6, 24.8, 24.8, 22.2, 14.4; HRMS-EI: calc. for C15H25O3B, 264.1897; found, 264.1899.
4,4,5,5-Tetramethyl-2-(1-phenylpentan-2-yl)-1,3,2-dioxaborolane (3t)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (500 mg, 91%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.19–7.25 (m, 4H, ArH), 7.11–7.16 (m, 1H, ArH), 2.71 (dd, J = 8.0 Hz, 13.56 Hz, 1H, 1/2ArCH2), 2.66 (dd, J = 8.0 Hz, 13.6 Hz, 1H, 1/2ArCH2), 1.45–1.48 (m, 5H, BCH + CHCH2 + CH3CH2), 1.16 (s, 6H, 2 × [1/2C(CH3)2]), 1.12 (s, 6H, 2 × [1/2C(CH3)2]), 0.89 (t, J = 8.0 Hz, 3H, CH2CH3); 13C NMR (100 MHz, CDCl3) δ 142.3, 128.8, 128.0, 125.5, 82.8, 37.4, 33.6, 24.8, 24.7, 22.3, 14.4; HRMS-EI: calc. for C17H27O2B, 274.2104; found, 274.2105.
3). Colorless oil (500 mg, 91%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.19–7.25 (m, 4H, ArH), 7.11–7.16 (m, 1H, ArH), 2.71 (dd, J = 8.0 Hz, 13.56 Hz, 1H, 1/2ArCH2), 2.66 (dd, J = 8.0 Hz, 13.6 Hz, 1H, 1/2ArCH2), 1.45–1.48 (m, 5H, BCH + CHCH2 + CH3CH2), 1.16 (s, 6H, 2 × [1/2C(CH3)2]), 1.12 (s, 6H, 2 × [1/2C(CH3)2]), 0.89 (t, J = 8.0 Hz, 3H, CH2CH3); 13C NMR (100 MHz, CDCl3) δ 142.3, 128.8, 128.0, 125.5, 82.8, 37.4, 33.6, 24.8, 24.7, 22.3, 14.4; HRMS-EI: calc. for C17H27O2B, 274.2104; found, 274.2105.
4,4,5,5-Tetramethyl-2-(1-(naphthalen-1-yl)pentan-2-yl)-1,3,2-dioxaborolane (3u)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (512 mg, 79%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 8.08 (d, J = 12.0 Hz, 1H, ArH), 7.81–7.84 (m, 1H, ArH), 7.67–7.69 (m, 1H, ArH), 7.43–7.51 (m, 2H, ArH), 7.34–7.40 (m, 2H, ArH), 3.20 (dd, J = 8.0 Hz, 14.0 Hz, 1H, 1/2ArCH2), 3.10 (dd, J = 8.0 Hz, 14.0 Hz, 1H, 1/2ArCH2), 1.28–1.59 (m, 5H, BCH + CHCH2 + CH3CH2), 1.17 (s, 6H, 2 × [1/2C(CH3)2]), 1.12 (s, 6H, 2 × [1/2C(CH3)2]), 0.91 (t, J = 8.0 Hz, 3H, CH2CH3); 13C NMR (100 MHz, CDCl3) δ 138.5, 134.0, 132.2, 128.7, 126.5, 126.5, 125.6, 125.3, 125.3, 124.3, 83.0, 344.5, 34.2, 24.9, 24.7, 22.5, 14.6; HRMS-EI: calc. for C21H29O2B, 324.2261; found, 324.2258.
3). Colorless oil (512 mg, 79%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 8.08 (d, J = 12.0 Hz, 1H, ArH), 7.81–7.84 (m, 1H, ArH), 7.67–7.69 (m, 1H, ArH), 7.43–7.51 (m, 2H, ArH), 7.34–7.40 (m, 2H, ArH), 3.20 (dd, J = 8.0 Hz, 14.0 Hz, 1H, 1/2ArCH2), 3.10 (dd, J = 8.0 Hz, 14.0 Hz, 1H, 1/2ArCH2), 1.28–1.59 (m, 5H, BCH + CHCH2 + CH3CH2), 1.17 (s, 6H, 2 × [1/2C(CH3)2]), 1.12 (s, 6H, 2 × [1/2C(CH3)2]), 0.91 (t, J = 8.0 Hz, 3H, CH2CH3); 13C NMR (100 MHz, CDCl3) δ 138.5, 134.0, 132.2, 128.7, 126.5, 126.5, 125.6, 125.3, 125.3, 124.3, 83.0, 344.5, 34.2, 24.9, 24.7, 22.5, 14.6; HRMS-EI: calc. for C21H29O2B, 324.2261; found, 324.2258.
4,4,5,5-Tetramethyl-2-(1-phenylpropan-2-yl)-1,3,2-dioxaborolane (3v)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (460 mg, 94%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.13–7.25 (m, 5H, ArH), 2.81 (dd, J = 8.0 Hz, 13.6 Hz, 1H, 1/2ArCH2), 2.54 (dd, J = 8.0 Hz, 13.6 Hz, 1H, 1/2ArCH2), 1.31–1.40 (m, 1H, BCH), 1.19 (s, 6H, 2 × [1/2C(CH3)2]), 1.18 (s, 6H, 2 × [1/2C(CH3)2]), 0.96 (d, J = 8.0 Hz, 3H, CHCH3); 13C NMR (125 MHz, CDCl3) δ 142.3, 128.9, 128.0, 125.6, 83.0, 39.1, 24.8, 15.3. These spectroscopic data correspond to reported data.7m
3). Colorless oil (460 mg, 94%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.13–7.25 (m, 5H, ArH), 2.81 (dd, J = 8.0 Hz, 13.6 Hz, 1H, 1/2ArCH2), 2.54 (dd, J = 8.0 Hz, 13.6 Hz, 1H, 1/2ArCH2), 1.31–1.40 (m, 1H, BCH), 1.19 (s, 6H, 2 × [1/2C(CH3)2]), 1.18 (s, 6H, 2 × [1/2C(CH3)2]), 0.96 (d, J = 8.0 Hz, 3H, CHCH3); 13C NMR (125 MHz, CDCl3) δ 142.3, 128.9, 128.0, 125.6, 83.0, 39.1, 24.8, 15.3. These spectroscopic data correspond to reported data.7m
2-(2,3-Dihydro-1H-inden-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3w)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (455 mg, 93%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.19–7.23 (m, 2H, ArH), 7.09–7.14 (m, 2H, ArH), 2.94–3.10 (m, 4H, 2ArCH2), 1.82–1.93 (m, 1H, BCH), 1.27 (s, 12H, 2C(CH3)2); 13C NMR (125 MHz, CDCl3) δ 144.5, 126.0, 124.3, 83.3, 35.2, 24.9. These spectroscopic data correspond to reported data.7e
3). Colorless oil (455 mg, 93%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.19–7.23 (m, 2H, ArH), 7.09–7.14 (m, 2H, ArH), 2.94–3.10 (m, 4H, 2ArCH2), 1.82–1.93 (m, 1H, BCH), 1.27 (s, 12H, 2C(CH3)2); 13C NMR (125 MHz, CDCl3) δ 144.5, 126.0, 124.3, 83.3, 35.2, 24.9. These spectroscopic data correspond to reported data.7e
4,4,5,5-Tetramethyl-2-(1,2,3,4-tetrahydro-2-naphthalenyl)-1,3,2-dioxaborolane (3x)
Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (482 mg, 94%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.04–7.09 (m, 4H, ArH), 2.72–2.89 (m, 4H, 2ArCH2), 1.99–2.06 (m, 1H, 1/2CHCH2), 1.60–1.71 (m, 1H, 1/2CHCH2), 1.29–1.38 (m, 1H, BCH), 1.26 (s, 6H, 2 × [1/2C(CH3)2]), 1.26 (s, 6H, 2 × [1/2C(CH3)2]); 13C NMR (125 MHz, CDCl3) δ 137.5, 137.1, 129.2, 129.0, 125.4, 83.2, 30.8, 29.8, 24.9, 24.8, 24.8. These spectroscopic data correspond to reported data.7e
3). Colorless oil (482 mg, 94%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.04–7.09 (m, 4H, ArH), 2.72–2.89 (m, 4H, 2ArCH2), 1.99–2.06 (m, 1H, 1/2CHCH2), 1.60–1.71 (m, 1H, 1/2CHCH2), 1.29–1.38 (m, 1H, BCH), 1.26 (s, 6H, 2 × [1/2C(CH3)2]), 1.26 (s, 6H, 2 × [1/2C(CH3)2]); 13C NMR (125 MHz, CDCl3) δ 137.5, 137.1, 129.2, 129.0, 125.4, 83.2, 30.8, 29.8, 24.9, 24.8, 24.8. These spectroscopic data correspond to reported data.7e
4,4,5,5-Tetramethyl-2-phen(1-D)ethyl-1,3,2-dioxaborolane (3a-D)
To a Schlenk tube equipped with a magnetic stir bar and charged with FeCl2 (5 mg, 0.04 mmol) was added THF (5 mL), followed by styrene (41 mg, 0.4 mmol), bis(pinacolato)diboron (150 mg, 0.6 mmol), tBuOK (53 mg, 0.48 mmol), CD3OD (72 mg, 2 mmol). The resulting solution was stirred at 65 °C for 12 h. The solution of the crude product was concentrated in vacuo, brine (5 mL) was added and the aqueous layer was extracted with EtOAc (3 × 10 mL). The combined organic layers were dried (Na2SO4) and the solvent removed under reduced pressure. Title compound was isolated by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100
1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Colorless oil (79 mg, 85%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.20–7.28 (m, 4H, ArH), 7.13–7.17 (m, 1H, ArH), 2.73 (t, J = 8.0 Hz, 1H, ArCH), 1.22 (s, 12H, 2C(CH3)2), 1.13 (d, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 144.4, 128.2, 128.0, 125.5, 83.1, 77.4, 77.2, 76.9, 29.8, 29.7, 29.5, 24.8. These spectroscopic data correspond to reported data.7m
3). Colorless oil (79 mg, 85%); 1H NMR (400 MHz, CDCl3, Me4Si) δ 7.20–7.28 (m, 4H, ArH), 7.13–7.17 (m, 1H, ArH), 2.73 (t, J = 8.0 Hz, 1H, ArCH), 1.22 (s, 12H, 2C(CH3)2), 1.13 (d, J = 8.0 Hz, 2H, BCH2); 13C NMR (125 MHz, CDCl3) δ 144.4, 128.2, 128.0, 125.5, 83.1, 77.4, 77.2, 76.9, 29.8, 29.7, 29.5, 24.8. These spectroscopic data correspond to reported data.7m
Conclusions
In conclusion, we have developed the first ferrous chloride catalyzed hydroboration of un-activated aryl alkenes with B2pin2 in the absence of ligands. The reaction proceeded smoothly in the presence of ferrous chloride, tBuOK and tBuOH, and the alkylboronates were obtained in high yield with high selectivity. Both substituted aryl ethylenes and internal alkenes gave good yield. The functional groups such as halogen, ester, amine, alkoxy, cyano etc. were tolerated well in the reaction. Employing air and moisture-stable B2pin2, using only 1 mol% of cheap and environment-friendly ferrous chloride, avoiding air and moisture-sensitive reductant and ligands make it a practical method for the preparation of alkylboronates.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 21576041 and 21231003). We also thank Prof. Baomin Wang for valuable discussions.References
- (a) H. C. Brown, Organic Synthesis via Boranes, Wiley, New York, 1975 Search PubMed; (b) M. Sato, N. Miyaura and A. Suzuki, Chem. Lett., 1989, 1405 CrossRef CAS; (c) S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544 CrossRef CAS; (d) Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, ed. D. G. Hall, Wiley-VCH, Weinheim, 2005 Search PubMed; (e) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed.
- Y. Yamamoto and N. Asao, Chem. Rev., 1993, 93, 2207 CrossRef CAS.
- (a) H. Chen, S. Schlecht, T. C. Semple and J. F. Hartwig, Science, 2000, 287, 1995 CrossRef CAS PubMed; (b) S. Shimada, A. S. Batsanov, J. A. K. Howard and T. B. Marder, Angew. Chem., Int. Ed., 2001, 40, 2168 CrossRef CAS; (c) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef CAS PubMed; (d) J. F. Hartwig, Acc. Chem. Res., 2012, 45, 864 CrossRef CAS PubMed; (e) Q. Li, C. W. Liskey and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 8755 CrossRef CAS PubMed.
- For selected reviews, see: (a) K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179 CrossRef CAS; (b) I. Beletskaya and A. Pelter, Tetrahedron, 1997, 53, 4957 CrossRef CAS; (c) I. Beletskaya and C. Moberg, Chem. Rev., 2006, 106, 2320 CrossRef CAS PubMed.
- (a) D. A. Evans, G. C. Fu and A. H. Hoveyda, J. Am. Chem. Soc., 1988, 110, 6917 CrossRef CAS; (b) D. A. Evans, G. C. Fu and A. H. Hoveyda, J. Am. Chem. Soc., 1992, 114, 6671 CrossRef CAS; (c) D. A. Evans, G. C. Fu and B. A. Anderson, J. Am. Chem. Soc., 1992, 114, 6679 CrossRef CAS; (d) S. A. Westcott, H. P. Blom, T. B. Marder and R. T. Baker, J. Am. Chem. Soc., 1992, 114, 8863 CrossRef CAS; (e) P. V. Ramachandran, M. P. Jennings and H. C. Brown, Org. Lett., 1999, 1, 1399 CrossRef CAS; (f) A. P. Luna, M. Bonin, L. Micouin and H.-P. Husson, J. Am. Chem. Soc., 2002, 124, 12098 CrossRef PubMed; (g) C. M. Crudden, Y. B. Hleba and A. C. Chen, J. Am. Chem. Soc., 2004, 126, 9200 CrossRef CAS PubMed; (h) A. M. Carroll, T. P. O'Sullivan and P. J. Guiry, Adv. Synth. Catal., 2005, 347, 609 CrossRef CAS; (i) J. Cipot, C. M. Vogel, R. McDonald, S. A. Westcott and M. Stradiotto, Organometallics, 2006, 25, 5965 CrossRef CAS; (j) D. R. Edwards, Y. B. Hleba, C. J. Lata, L. A. Calhoun and C. M. Crudden, Angew. Chem., Int. Ed., 2007, 46, 7799 CrossRef CAS PubMed; (k) K. Endo, M. Hirokami and T. Shibata, Organometallics, 2008, 27, 5390 CrossRef CAS; (l) T. Shiomi, T. Adachi, K. Toribatake, L. Zhou and H. Nishiyama, Chem. Commun., 2009, 5987 RSC; (m) B. Nguyen and J. M. Brown, Adv. Synth. Catal., 2009, 351, 1333 CrossRef CAS; (n) C. J. Lata and C. M. Crudden, J. Am. Chem. Soc., 2010, 132, 131 CrossRef CAS PubMed; (o) S. M. Smith and J. M. Takacs, Org. Lett., 2010, 12, 4612 CrossRef CAS PubMed.
- (a) J. A. Brinkman, T. T. Nguyen and J. R. Sowa, Org. Lett., 2000, 2, 981 CrossRef CAS PubMed; (b) Y. Yamamota, R. Fujikawa, T. Umemoto and N. Miyaura, Tetrahedron, 2004, 60, 10695 CrossRef; (c) J. A. Melanson, C. M. Vogels, A. Decken and S. A. Westcott, Inorg. Chem. Commun., 2010, 13, 1396 CrossRef CAS; (d) C. Mazet and D. Gérard, Chem. Commun., 2011, 47, 298 RSC; (e) G. M. Lee, C. M. Vogels, A. Decken and S. A. Westcott, Eur. J. Inorg. Chem., 2011, 2433 CrossRef CAS; (f) N. M. Hunter, C. M. Vogels, A. Decken, A. Bell and S. A. Westcott, Inorg. Chim. Acta, 2011, 365, 408 CrossRef CAS.
- (a) J.-E. Lee and J. Yun, Angew. Chem., Int. Ed., 2008, 47, 145 CrossRef CAS PubMed; (b) D. Noh, H. Chea, J. Ju and J. Yun, Angew. Chem., Int. Ed., 2009, 48, 6062 CrossRef CAS PubMed; (c) X. Feng and J. Yun, Chem. Commun., 2009, 6577 RSC; (d) H.-S. Sim, X. Feng and J. Yun, Chem.–Eur. J., 2009, 15, 1939 CrossRef CAS PubMed; (e) Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 3160 CrossRef CAS PubMed; (f) M. Gao, S. B. Thorpe and W. L. Santos, Org. Lett., 2009, 11, 3478 CrossRef CAS PubMed; (g) J. A. Schiffner, K. Müther and M. Oestreich, Angew. Chem., Int. Ed., 2010, 49, 1194 CrossRef CAS PubMed; (h) Y. Sasaki, C. Zhong, M. Sawamura and H. Ito, J. Am. Chem. Soc., 2010, 132, 1226 CrossRef CAS PubMed; (i) R. Corberán, N. W. Mszar and A. H. Hoveyda, Angew. Chem., Int. Ed., 2011, 50, 7079 CrossRef PubMed; (j) X. Feng, H. Jeon and J. Yun, Angew. Chem., Int. Ed., 2013, 52, 3989 CrossRef CAS PubMed; (k) K. Semba, M. Shinomiya, T. Fujihara, J. Terao and Y. Tsuji, Chem.–Eur. J., 2013, 19, 7125 CrossRef CAS PubMed; (l) N. Matsuda, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2013, 135, 4934 CrossRef CAS PubMed; (m) Y. Wen, J. Xie, C. Deng and C. Li, J. Org. Chem., 2015, 80, 4142 CrossRef CAS PubMed.
- (a) M. Zaidlewica and J. Meller, Tetrahedron Lett., 1997, 38, 7279 CrossRef; (b) J. V. Obligacion and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 19107 CrossRef CAS PubMed; (c) L. Zhang, Z. Zuo, X. Wan and Z. Huang, J. Am. Chem. Soc., 2014, 136, 15501 CrossRef CAS PubMed; (d) L. Zhang, Z. Zuo, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2014, 53, 2696 CrossRef CAS PubMed; (e) W. N. Palmer, T. Diao, I. Pappas and P. J. Chirik, ACS Catal., 2015, 5, 622 CrossRef CAS.
- (a) C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 528 CrossRef CAS PubMed; (b) H. Ito and K. Kubota, Org. Lett., 2012, 14, 890 CrossRef CAS PubMed; (c) A. S. Dudnik and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 10693 CrossRef CAS PubMed; (d) T. C. Atack, R. M. Lecker and S. P. Cook, J. Am. Chem. Soc., 2014, 136, 9521 CrossRef CAS PubMed; (e) Z.-Q. Zhang, C.-T. Yang, L.-J. Liang, B. Xiao, X. Lu, J.-H. Liu, Y.-Y. Sun, T. B. Marder and Y. Fu, Org. Lett., 2014, 16, 6342 CrossRef CAS PubMed; (f) R. B. Bedford, P. B. Brenner, E. Carter, T. Gallagher, D. M. Murphy and D. R. Pye, Organometallics, 2014, 33, 5940 CrossRef CAS; (g) W. K. Chow, O. Y. Yuen, P. Y. Choy, C. M. So, C. P. Lau, W. T. Wong and F. Y. Kwong, RSC Adv., 2013, 3, 12518 RSC; (h) J. H. Kim and Y. K. Chung, RSC Adv., 2014, 4, 39755 RSC; (i) X.-F. Zhou, Y.-D. Wu, J.-J. Dai, Y.-J. Li, Y. Huang and H.-J. Xu, RSC Adv., 2015, 5, 46672 RSC.
- Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, 2008 Search PubMed.
- (a) S. C. Bart, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2004, 126, 13794 CrossRef CAS PubMed; (b) K. Kamata, A. Suzuki, Y. Nakai and H. Nakazawa, Organometallics, 2012, 31, 3825 CrossRef CAS; (c) A. M. Tondreau, C. C. H. Atienza, J. M. Darmon, C. Milsmann, H. M. Hoyt, K. J. Weller, S. A. Nye, K. M. Lewis, J. Boyer, J. G. P. Delis, E. Lobkovsky and P. J. Chirik, Organometallics, 2012, 31, 4886 CrossRef CAS; (d) A. M. Tondreau, C. C. H. Atienza, K. J. Weller, S. A. Nye, K. M. Lewis, J. G. P. Delis and P. J. Chirik, Science, 2012, 335, 567 CrossRef CAS PubMed.
- (a) D. Srimani, Y. Diskin-Posner, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2013, 52, 14131 CrossRef CAS PubMed; (b) Y. Li, S. Yu, X. Wu, J. Xiao, W. Shen, Z. Dong and J. Gao, J. Am. Chem. Soc., 2014, 136, 4031–4039 CrossRef CAS PubMed.
- (a) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS PubMed; (b) A. Fürstner, Angew. Chem., Int. Ed., 2009, 48, 1364 CrossRef PubMed; (c) M. Guisán-Ceinos, F. Tato, E. Buñuel, P. Calle and D. J. Cárdenas, Chem. Sci., 2013, 4, 1098 RSC; (d) K. Li, G. Tan, J. Huang, F. Song and J. You, Angew. Chem., Int. Ed., 2013, 52, 12942 CrossRef CAS PubMed.
- (a) A. Varela-Álvarez and D. G. Musaev, Chem. Sci., 2013, 4, 3758 RSC; (b) T. Hashimoto, D. Hirose and T. Taniguchi, Angew. Chem., Int. Ed., 2014, 53, 2730 CrossRef CAS PubMed; (c) Q. Gu, H. H. A. Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868 CrossRef CAS PubMed; (d) B. M. Monks, E. R. Fruchey and S. P. Cook, Angew. Chem., Int. Ed., 2014, 53, 11065 CrossRef CAS PubMed; (e) T. Matsubara, S. Asako, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2014, 136, 646 CrossRef CAS PubMed; (f) E. R. Fruchey, B. M. Monks and S. P. Cook, J. Am. Chem. Soc., 2014, 136, 13130 CrossRef CAS PubMed.
- (a) W.-T. Wei, M.-B. Zhou, J.-H. Fan, W. Liu, R.-J. Song, Y. Liu, M. Hu, P. Xie and J.-H. Li, Angew. Chem., Int. Ed., 2013, 52, 3638 CrossRef CAS PubMed; (b) J. M. Hoyt, K. T. Sylvester, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 4862 CrossRef CAS PubMed; (c) E. Bernoud, P. Oulié, R. Guillot, M. Mellah and J. Hannedouche, Angew. Chem., Int. Ed., 2014, 53, 4930 CrossRef CAS PubMed.
- (a) T. Xu, C. W. Cheung and X. Hu, Angew. Chem., Int. Ed., 2014, 53, 4910 CrossRef CAS PubMed; (b) M. Iwasaki, T. Fujii, K. Nakajima and Y. Nishihara, Angew. Chem., Int. Ed., 2014, 53, 13880 CrossRef CAS PubMed; (c) M. Haberberger and S. Enthaler, Chem.–Asian J., 2013, 8, 50 CrossRef CAS PubMed; (d) V. S. Rawat and B. Sreedhar, Synlett, 2014, 25, 1132 CrossRef.
- J. Y. Wu, B. Moreau and T. Ritter, J. Am. Chem. Soc., 2009, 131, 12915 CrossRef CAS PubMed.
- (a) L. Zhang, D. Peng, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2013, 52, 3676 CrossRef CAS PubMed; (b) L. Zhang and Z. Huang, Synlett, 2013, 24, 1745 CrossRef CAS; (c) Y. Cao, Y. Zhang, L. Zhang, D. Zhang, X. Leng and Z. Huang, Org. Chem. Front., 2014, 1, 1101 RSC.
- M. D. Greenhalgh and S. P. Thomas, Chem. Commun., 2013, 49, 11230 RSC.
- J. V. Obligacion and P. J. Chirik, Org. Lett., 2013, 15, 2680 CrossRef CAS PubMed.
- J. Chen, T. Xi and Z. Lu, Org. Lett., 2014, 16, 6452 CrossRef CAS PubMed.
- K.-N. T. Tseng, J. W. Kampf and N. K. Szymczak, ACS Catal., 2015, 5, 411 CrossRef CAS.
- J. Zheng, J.-B. Sortais and C. Darcel, ChemCatChem, 2014, 6, 763 CrossRef CAS.
- A. Bonet, C. Sole, H. Gulyás and E. Fernández, Chem.–Asian J., 2011, 6, 1011 CrossRef CAS PubMed.
- (a) L. Dang and Z. Lin, Organometallics, 2008, 27, 4443 CrossRef CAS; (b) A. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás and E. Fernández, Angew. Chem., Int. Ed., 2011, 50, 7158 CrossRef CAS PubMed; (c) E. Yamamoto, S. Ukigai and H. Ito, Chem. Sci., 2015, 6, 2943 RSC; (d) N. Nakagawa, T. Hatakeyama and M. Nakamura, Chem.–Eur. J., 2015, 21, 4257 CrossRef CAS PubMed.
- (a) I. A. Barker, J. E. Harfi, K. Adlington, S. M. Howdle and D. J. Irvine, Macromolecules, 2012, 45, 9258 CrossRef CAS; (b) G. Baccolini, C. Delpivo and G. Micheletti, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 1291 CrossRef CAS.
- P. Kovacic and N. O. Brace, J. Am. Chem. Soc., 1954, 76, 5491 CrossRef CAS.
- K. Endo, T. Ohkubo, T. Ishioka and T. Shibata, J. Org. Chem., 2012, 77, 4826 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14869c |
| This journal is © The Royal Society of Chemistry 2015 |