Development of antibacterial polyacrylonitrile membrane modified with a covalently immobilized lysozyme
Wei Liua,
Minhua Caia,
Yuegui Hea,
Shuai Wanga,
Jinwang Zhengb and
Xiaoping Xu*a
aResearch Institute of Photocatalysis, College of Chemistry, Fuzhou University, Fuzhou 350108, China. E-mail: xu@fzu.edu.cn; Tel: +86 13067237982
bShanghai Tofflon Science and Technology Co., Ltd., Shanghai, China
First published on 18th September 2015
Abstract
A novel antibacterial polyacrylonitrile (PAN) membrane covalently immobilized with lysozyme was prepared. First, the virginal PAN membranes were prepared via the classic immersion precipitation method. After modification with NaOH, HCl, ethylenediamine (EDA), the lysozyme was covalently immobilized onto the surface of the PAN membranes by glutaraldehyde. The chemical compositions of virginal and modified membranes were characterized by Fourier transform infrared spectroscopy (FT-IR) and Energy Dispersion X-ray (EDX). The morphology and performance of the immobilized membranes were characterized by Scanning Electronic Microscopy (SEM), filtration performance measurements, the amount of bonded lysozyme, lysozyme activity measurement and flow cytometry method. The antibacterial tests confirmed that the immobilized lysozyme membrane displayed an excellent antibacterial performance against Staphylococcus aureus (S. aureus).
1. Introduction
In recent years, membrane separation has been widely used in water treatment, food, pharmaceutical, and biotechnological industries. In all polymer membranes, the polyacrylonitrile (PAN) membrane occupies the main part owing to its good solvent resistance, sufficient chemical stability, excellent mechanical properties, and superior thermal stability.1 However, membrane fouling will shorten the membrane lifetime, reduce membrane flux, increase energy costs and bio-deteriorating of membrane structures.2 In food separation and water treatment, membrane fouling caused by the undesirable accumulation of microorganisms (mainly bacteria) is known as bio-fouling. And the build-up of biofilm is considered as a major obstacle in membrane separation. To decrease the bio-fouling due to biofilm by bacteria on the membrane surface, antibacterial treatment of the membrane surface with antibacterial agents is desired. Actually, a series of investigations3,4 and antibacterial agents have been employed to construct bactericidal membranes, including N-halamine compounds,5–7 silver ions/nanoparticles (AgNPs),8–10 various polycations,11–13 and enzymes.14–16 Unfortunately, these methods either would lose their antibacterial properties as the antibacterial substance got released into the surrounding or the whole process was cumbersome, environmentally-unfriendly. With the growing public health awareness of the pathogenic effects, and stain formations caused by microorganisms, there is an increasing need for producing an antibacterial membrane that can not only eliminate the bacterial or even kill the bacterial, but also can keep excellent antibacterial properties after multiple uses.Lysozyme is widely distributed in nature and well known for its antibacterial activity. It can break down bacterial cell walls via catalyzing the hydrolysis of 1,4-beta-linkages between N-acetyl-D-glucosamine and N-acetylmuramic acid residues in chitodextrins, which increased the permeability of bacteria and caused bacteria to burst.17 Nevertheless, lysozyme as a soluble enzyme was very unstable and retained its activity only for a short period of time. Therefore, a lot of researches have focused on the conduct of molecular proteins that are immobilized on a solid support.18,19 Howell et al. improved the flux of ultrafiltration membrane by attaching enzyme onto UF membrane which can hinder the gel formation.20 Shi et al. developed anti-fouling and self-cleaning polyethersulfone membrane through immobilization of trypsin.21 Compared with the free enzyme, immobilized enzyme showed more stability and more robust resistance to environmental changes. In addition, the immobilized enzyme system provided unique advantages in process control and continuous operation, which made the process more economical.22
In this study, virginal PAN membranes were prepared by the immersion precipitation phase-inversion method. After modification, lysozyme was covalently immobilized onto the surface of PAN membranes by glutaraldehyde. Morphologies, structures, water flux and antimicrobial efficacies (against S. aureus) of the membranes were investigated. The result indicated the activity of immobilized lysozyme was relatively high and could effectively prevent formation of biofilms. The highly antibacterial membranes have potential application in water treatment and food manufacturing.
2. Experimental
2.1 Materials
Polyacrylonitrile (PAN) with an average molecular weight of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 as the membrane-forming polymer was acquired from Sigma Chemical Co. Ltd. Lysozyme with a molecular weight of 14.4 kDa was supplied by Shanghai Sangon Biological Engineering Technology Service Co. Ltd. N-Methyl-2-pyrrolidone (NMP), phosphoric acid, ethylenediamine (EDA), glutaraldehyde, sodium hydroxide, hydrochloric acid and other chemicals were purchased from Xilong Chemical Co. Ltd. All chemicals were used without further purification. S. aureus was preserved by our laboratory.
000 g mol−1 as the membrane-forming polymer was acquired from Sigma Chemical Co. Ltd. Lysozyme with a molecular weight of 14.4 kDa was supplied by Shanghai Sangon Biological Engineering Technology Service Co. Ltd. N-Methyl-2-pyrrolidone (NMP), phosphoric acid, ethylenediamine (EDA), glutaraldehyde, sodium hydroxide, hydrochloric acid and other chemicals were purchased from Xilong Chemical Co. Ltd. All chemicals were used without further purification. S. aureus was preserved by our laboratory.
2.2 Preparation of virginal PAN membrane
The virginal PAN membranes were prepared via the classic immersion precipitation method from a NMP solution containing 16 wt% PAN powder and 4 wt% pore-forming agent H3PO4. The casting solution was stirred at 70 °C for 4 h and kept another 8 h at ambient temperature to completely release air bubbles. Subsequently, the solution was cast on the glass plate (cleaned with acetone) with a casting knife. Then the glass plate was immediately immersed into a coagulation bath of deionized water at 25 °C. After the primary membranes were peeled off from the substrate, the obtained membranes were left in deionized water overnight to completely remove the residual solvent.23 The virginal membranes (V-PAN) were stored in deionized water for further utilization.2.3 Surface modification of PAN membrane
For modification, the PAN membranes with 16 cm2 surface area were first immersed into a 1 M NaOH solution and then the solution was left in a constantly shaking water bath at 40 °C for 1 hour until its color turned into a wine-red. The membranes were then rinsed with deionized water until neutral pH was reached and then immersed in to a 10% (v/v) HCl solution at room temperature for 2 h.24 After re-rinsing with water until neutral pH was reached again, the modified membranes (M-PAN) were immersed in 150 cm3 10% (w/w) solution of ethylenediamine for 1 h at room temperature. Finally, the gained membranes were taken out and washed thoroughly with deionized water to remove the unreacted ethylenediamine.2.4 Immobilization of lysozyme on modified membrane
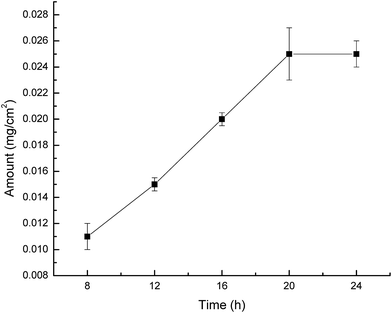
The modified membranes (16 cm2) were equilibrated with 10 mL of sodium phosphate buffer (pH 7.0) for 4 h. The equilibrated membranes were transferred to 10% (v/v) solution of glutaraldehyde for 1 h at 4 °C, then the membranes were washed 3 times with 0.1 M sodium phosphate buffer (pH 7.0) to remove the excess glutaraldehyde.25 The activated membranes were treated with lysozyme solution (1 g L−1) for up to 20 h at 4 °C.26 Then immobilized membranes (I-PAN) were taken out and thoroughly rinsed with deionized water and 0.1 M solution of phosphate buffer. The preparation process of immobilized membrane (I-PAN) was shown in Fig. 1.2.5 Separation performance of membranes
A laboratory scale cross flow membrane system was used to characterize the performance of V-PAN, M-PAN, and I-PAN. The membranes were immersed in deionized water overnight at ambient temperature before filtration. Deionized water was selected as feed solution. The sample membrane with an effective area of 10.48 cm2 was installed on the membrane cell. The system was pre-compacted with deionized water at 0.2 MPa for 3 h to reach a steady state, followed by measuring water fluxes at pressures ranging from 0.08 to 0.16 MPa. And the feed flow rate 1.0 L min−1. The water flux (J) was calculated using eqn (1)
 | (1) |
For bio-fouling investigations, after the pure water permeability J0 was measured, the flux for bacterial suspension (S. aureus, 105 cells per mL) was measured at the same pressure. The filtration of the bacterial suspension was continued for 9 h and then the membrane was immersed in citric acid solution (pH 4.0) for 1 h and backwashed thoroughly with deionized water for 0.5 h. The water flux of the cleaned membranes JR was measured again. Such a cycle of filtration was carried out continuously for three times and the operating pressure was 0.1 MPa for the whole process. In order to evaluate the anti-fouling property of membranes, the flux recovery ratio (FRR) was calculated as following eqn (2):
 | (2) |
2.6 Immobilized lysozyme estimation
| Mb = C0V0M − (C1V1 + C2V2)M | (3) |
The hydrolytic activity of the immobilized lysozyme can be calculated using the follow eqn (4):
| U/cm2 = ΔOD450 × 1000/S | (4) |
2.7 Membrane characterization
2.8 Determination of antibacterial properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. And after removal of supernatant, the cells were washed twice with sterile phosphate buffered solution (PBS, pH 7.4). Then the bacteria cells were re-suspended and diluted to 106 cells per mL using the standard serial dilution method (based on standard calibration with the assumption that the optical density of 1.0 at 540 nm is equivalent to approximately 109 cells per mL).29
000 rpm for 10 min. And after removal of supernatant, the cells were washed twice with sterile phosphate buffered solution (PBS, pH 7.4). Then the bacteria cells were re-suspended and diluted to 106 cells per mL using the standard serial dilution method (based on standard calibration with the assumption that the optical density of 1.0 at 540 nm is equivalent to approximately 109 cells per mL).29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events were detected each run and the rate of samples was 1000 events per second. The samples were stained with Propidium Iodide (PI) because PI binds to DNA and cannot cross an intact cell.30 The antibacterial efficiency (Eb) was represented by detecting the percent of broken cells. The antibacterial efficiency was measured from eqn (5):
000 events were detected each run and the rate of samples was 1000 events per second. The samples were stained with Propidium Iodide (PI) because PI binds to DNA and cannot cross an intact cell.30 The antibacterial efficiency (Eb) was represented by detecting the percent of broken cells. The antibacterial efficiency was measured from eqn (5):| Eb = (W2 − W1) × 100% | (5) |
3. Results and discussion
3.1 Surface chemical composition of the membranes
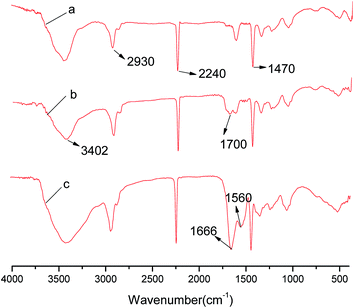
V-PAN, M-PAN, and I-PAN are virginal PAN membrane, PAN membrane modified NaOH, HCl, PAN membrane immobilized lysozyme respectively. The V-PAN, M-PAN, and I-PAN were subjected to FT-IR analysis. The FT-IR spectrum of V-PAN was shown in Fig. 2a. The spectrum of V-PAN shows characteristic peaks at 2930, 2240, 1470 cm−1 were stretching vibration band of methylene (–CH2–), stretching vibration band of nitriles (–CN–), and bending vibration band of methylene (–CH2–), respectively. The characteristic spectra of virginal PAN was similar to a previous research of enzymatic surface modification of polyacrylonitrile and its copolymers.31,32 In the spectrum of Fig. 2b, there are two prominent peaks in the 3402 cm−1 region and 1699–1711 cm−1 region respectively. This phenomenon was most likely to be ascribed to the presence of O–H bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of carboxylic acids in NaOH treated membrane. These two stretching peaks indicated the presence of –COOH in membrane after treated by NaOH. The two common bands of lysozyme 1658 and 1519 cm−1 belong to the amide I and amide II peaks have appeared in Fig. 2c, which corresponded to stretching vibrations of C
O bond of carboxylic acids in NaOH treated membrane. These two stretching peaks indicated the presence of –COOH in membrane after treated by NaOH. The two common bands of lysozyme 1658 and 1519 cm−1 belong to the amide I and amide II peaks have appeared in Fig. 2c, which corresponded to stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and coupling of bending of N–H bond and stretching of C–N bond, respectively. However these peaks migrated to 1666 and 1560 cm−1 by the influence of the original amide group.33,34 The appearance of these characteristic peaks indicated that lysozyme was immobilized on the PAN membranes by covalent bonding.
O bond and coupling of bending of N–H bond and stretching of C–N bond, respectively. However these peaks migrated to 1666 and 1560 cm−1 by the influence of the original amide group.33,34 The appearance of these characteristic peaks indicated that lysozyme was immobilized on the PAN membranes by covalent bonding.
The changes in elemental composition between different membranes were analyzed by EDX. As shown in Table 1, the C element accounted for 63.98% (wt) in V-PAN and 63.65% in M-PAN, then increased to 66.07% (wt) in I-PAN. The EDX shows that no S element is existed in V-PAN and M-PAN. The S element accounted for 0.08% (wt) in I-PAN because of the immobilized lysozyme. These changes further confirmed the successful grafting of lysozyme onto the PAN membrane.
| Element | V-PAN | M-PAN | I-PAN |
|---|---|---|---|
| C | 63.98 | 63.65 | 66.07 |
| O | 19.34 | 11.83 | 8.50 |
| N | 16.34 | 24.20 | 25.17 |
| S | 0.00 | 0.00 | 0.08 |
| P | 0.34 | 0.32 | 0.18 |
3.2 SEM analysis
The SEM images of the cross section of V-PAN, M-PAN, and I-PAN were presented in Fig. 3. It is apparent that all the membranes have typical asymmetric structures, which consist of a thin dense layer and a support layer with finger-like structure. The diameters of finger-like structure in modified/immobilized membrane were larger than its counterpart in V-PAN. These changes may be attributed to the damage in the process of modification by sodium hydroxide treatment.35 Further, in the last step of immobilization of lysozyme, there was no evident change in the cross-sectional morphology of the M-PAN/I-PAN, which can be verified in the Fig. 3b and c.3.3 Membrane filtration performance
A cross flow membrane system was used to characterize the performance of the V-PAN, M-PAN and I-PAN. Fig. 4 illustrates the pure water flux through the V-PAN, M-PAN and I-PAN membranes at different feed pressures. As the applied pressure increased, the pure water flux increased linearly. M-PAN had relatively higher pure water flux than V-PAN and I-PAN. This change can be attributed to that the finger-hole became a little bigger35 and the surface hydrophilicity of V-PAN was enhanced after NaOH modification. Some enzyme was embedded in the holes when lysozyme was immobilized on the PAN membrane, which resulted in a reduction in the water permeability.As shown in Fig. 5, the flux of membrane decreased sharply in the initial few hours of operation. It is proposed that some S. aureus cells in the feed were deposited or adsorbed on the surface (biofilms formation). The flux recovery ratio (FRR) by cleaning with deionized water was analyzed for tested membranes. After first cycle, the FRR were 86.9%, 85.7%, and 93.1% for the V-PAN, M-PAN, I-PAN, respectively. The FRR decreased for both tested membranes with the increase in the operation time. The FRR of the second cycle were 75.4%, 77.8% and 86.2% for the V-PAN, M-PAN, I-PAN, respectively. Compared the data of V-PAN and M-PAN, the litter improvement of flux recovery may be attributed to enhance of hydrophility due the NaOH modification. Although immobilized membrane showed a fraction flux loss due to the fouling on the membrane surface, their higher recovery ratio proved that their absorbed fouling layers are relatively loose and easily removable. The phenomenon can be attributed to the higher hydrophility and reduce the biofilm formation of immobilized membrane due to lysozyme.
3.4 The amount and activity of the immobilized lysozyme
The preservation stability of the lysozyme immobilized on the membrane was also investigated. After preservation in 0.1 M PBS (pH 6.8) solution for 3 months, the immobilized lysozyme lost 24% of their initial activity. Some lysozyme was denatured and lost activity via hydrolysis though it was covalently immobilized.
3.5 Determination of antibacterial properties
The viable and broken cell counts of bacteria were measured by flow cytometry method. At the predetermined time, 0.2 mL of bacteria culture was taken from sample solution and decimal serial dilutions with PBS were repeated with each initial sample. In total, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events were detected each run and the rate of samples was 1000 events per second. The antibacterial efficiencies (Eb) of the V-PAN/I-PAN were presented in Fig. 7. In the image, X-axis refers to signal intensity of PI while Y-axis is number of cells. After being filtered by I-PAN, the number of intact of S. aureus was significantly less than those in the V-PAN. The percent of broken cells of S. aureus in control group was 19.3%, which was presented in Fig. 7a. When cells are in the exponential growth phase, it is a normal phenomenon that some cells would apoptosis. The percent of broken cells of S. aureus in experiment group was 56.6%, which was presented in Fig. 7b. The change of the percent of broken cells indicated that the immobilized lysozyme having a strong bacterial-killing capacity against S. aureus with an antibacterial efficiency (Eb) of 37.3%. This study showed that the immobilized membrane had the potential to reduce bio-fouling in water treatment and food separation.
000 events were detected each run and the rate of samples was 1000 events per second. The antibacterial efficiencies (Eb) of the V-PAN/I-PAN were presented in Fig. 7. In the image, X-axis refers to signal intensity of PI while Y-axis is number of cells. After being filtered by I-PAN, the number of intact of S. aureus was significantly less than those in the V-PAN. The percent of broken cells of S. aureus in control group was 19.3%, which was presented in Fig. 7a. When cells are in the exponential growth phase, it is a normal phenomenon that some cells would apoptosis. The percent of broken cells of S. aureus in experiment group was 56.6%, which was presented in Fig. 7b. The change of the percent of broken cells indicated that the immobilized lysozyme having a strong bacterial-killing capacity against S. aureus with an antibacterial efficiency (Eb) of 37.3%. This study showed that the immobilized membrane had the potential to reduce bio-fouling in water treatment and food separation.
4. Conclusions
In this study, a PAN ultrafiltration membranes prepared via the classic immersion precipitation method was modified by immobilized lysozyme on the surface of membranes, resulting in a novel PAN-immobilized membrane with anti-bacterial property. The membrane surface morphology, separation performance, immobilized lysozyme activity, and antibacterial activity were investigated. Compared to the virginal membrane, the membrane exhibited a moderate flux and a high antibacterial activity. In addition, the antibacterial activity analysis showed that the number of intact cells of S. aureus after filtered by the immobilized membrane was significantly less than those filtered by the virginal membrane, with an antibacterial efficiency of 37.3%. Therefore, membrane with effective antibacterial performances has been prepared, which will have potential applications beyond water treatment or food manufacturing.Acknowledgements
The authors gratefully acknowledge financial assistance by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20103514110002) and National Natural Science Foundation of China (No. J1103303). The authors also gratefully acknowledge professor of Zhang Guoliang for providing to improve the language of manuscript.References
- X. N. Chen, L. S. Wan, Q. Y. Wu, S. L. Zhi and Z. K. Xu, Mineralized polyacrylonitrile-based ultrafiltration membranes with improved water flux and rejection towards dye, J. Membr. Sci., 2013, 441, 112–119 CrossRef CAS PubMed.
- Y. F. Yang, H. Q. Hu, Y. Li, L. S. Wan and Z. K. Xu, Membrane surface with antibacterial property by grafting polycation, J. Membr. Sci., 2011, 376, 132–141 CrossRef CAS PubMed.
- Y. Mei, C. Yao, K. Fan and X. S. Li, Surface modification of polyacrylonitrile nanofibrous membranes with superior antibacterial and easy-cleaning properties through hydrophilic flexible spacers, J. Membr. Sci., 2012, 417–418, 20–27 CrossRef CAS PubMed.
- J. Xu, X. S. Feng, P. P. Chen and C. J. Gao, Development of an antibacterial copper(II)-chelated polyacrylonitrile ultrafiltration membrane, J. Membr. Sci., 2012, 413–414, 62–69 CrossRef CAS PubMed.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, N-Halamine Biocidal Coatings via a Layer-by-Layer Assembly Technique, Langmuir, 2011, 27, 4091–4097 CrossRef CAS PubMed.
- J. Jang and Y. Kim, Fabrication of monodisperse silica-polymer core–shell nanoparticles with excellent antimicrobial efficacy, Chem. Commun., 2008, 4016–4018 RSC.
- A. Dong, Q. Zhang, T. Wang, W. W. Wang, F. Q. Liu and G. Gao, Immobilization of Cyclic N-Halamine on Polystyrene-Functionalized Silica Nanoparticles: Synthesis, Characterization, and Biocidal Activity, J. Phys. Chem. C, 2010, 114, 17298–17303 CAS.
- K. Zodrow, L. Brunet, S. Mahendra, D. Li, A. N. Zhang, Q. L. Li and P. J. Alvarez, Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal, Water Res., 2009, 43, 715–723 CrossRef CAS PubMed.
- D. G. Yu, M. Y. Teng, W. L. Chou and M. C. Yang, Characterization and inhibitory effect of antibacterial PAN-based hollow fiber loaded with silver nitrate, J. Membr. Sci., 2003, 225, 115–123 CrossRef CAS PubMed.
- W. L. Chou, D. G. Yu and M. C. Yang, The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment, Polym. Adv. Technol., 2005, 16, 600–607 CrossRef CAS PubMed.
- E. Kenawy, S. D. Worley and R. Broughton, The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review, Biomacromolecules, 2007, 8, 1359–1384 CrossRef CAS PubMed.
- J. Haldar, D. Q. An, D. C. L. Alvarez, J. Z. Chen and A. M. Klibanov, Polymeric coatings that inactivate both influenza virus and pathogenic bacteria, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 17667–17671 CrossRef CAS PubMed.
- J. C. Tiller, C. J. Liao, K. Lewis and A. M. Klibanov, Designing surfaces that kill bacteria on contact, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5981–5985 CrossRef CAS PubMed.
- A. K. Muszanska, H. J. Busscher, A. Herrmann, H. C. van der Mei and W. Norde, Pluronic–lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating, Biomacromolecules, 2011, 32, 6333–6341 CAS.
- A. Caro, V. Humblot, C. Méthivier, M. Minier, M. Salmain and C. M. Pradier, Grafting of Lysozyme and/or Poly(ethylene glycol) to Prevent Biofilm Growth on Stainless Steel Surfaces, J. Phys. Chem. B, 2009, 113, 2101–2109 CrossRef CAS PubMed.
- R. C. Pangule, S. J. Brooks, C. Z. Dinu, S. S. Bale, S. L. Salmon and G. Y. Zhu, Antistaphylococcal Nanocomposite Films Based on Enzyme–Nanotube Conjugates, ACS Nano, 2010, 4, 3993–4000 CrossRef CAS PubMed.
- Z. X. Lian, Z. S. Ma, J. Wei and H. Liu, Preparation and characterization of immobilized lysozyme and evaluation of its application in edible coatings, Process Biochem., 2012, 47, 201–208 CrossRef CAS PubMed.
- J. M. Park, M. Kim, H. S. Park, A. Jang, J. Min and Y. H. Kim, Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications, Int. J. Biol. Macromol., 2013, 54, 37–43 CrossRef CAS PubMed.
- J. V. Edwards, N. T. Prevost, B. Condon, A. French and Q. L. Wu, Immobilization of lysozyme–cellulose amide-linked conjugates on cellulose I and II cotton nanocrystalline preparations, Cellulose, 2012, 19, 495–506 CrossRef CAS.
- J. A. Howell and O. Velicangil, Theoretical Considerations of Membrane Fouling and Its Treatment with Immobilized Enzymes for Protein Ultrafiltration, J. Appl. Polym. Sci., 1982, 27, 21–32 CrossRef CAS PubMed.
- Q. Shi, Y. L. Su, X. Ning, W. J. Chen, J. M. Peng and Z. Y. Jiang, Trypsin-enabled construction of anti-fouling and self-cleaning polyethersulfone membrane, Bioresour. Technol., 2011, 102, 647–651 CrossRef CAS PubMed.
- J. K. Park, M. Kim, H. S. Park, A. Jang, J. Min and Y. H. Kim, Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications, Int. J. Biol. Macromol., 2013, 54, 37–43 CrossRef CAS PubMed.
- M. Z. Li, J. H. Li, J. H. Shao, J. Miao, J. Wang, Q. Q. Zhang and X. P. Xu, Grafting zwitterionic brush on the surface of PVDF membrane using physisorbed free radical grafting technique, J. Membr. Sci., 2012, 405–406, 141–148 CrossRef CAS PubMed.
- F. Y. Mahlicli, S. A. Altinkaya and Y. Yurekli, Preparation and characterization of polyacrylonitrile membranes modified with polyelectrolyte deposition for separating similar sized proteins, J. Membr. Sci., 2012, 415–416, 383–390 CrossRef PubMed.
- T. Godjevargova and K. Gabrovska, Influence of matrix on external mass transfer resistance in immobilized urease membranes, Enzyme Microb. Technol., 2006, 38, 338–342 CrossRef CAS PubMed.
- J. K. Park, M. Kim, H. S. Park, A. Jang, J. Min and Y. H. Kim, Immobilization of lysozyme-CLE onto electrospun chitosan nanofiber for effective antibacterial applications, Int. J. Biol. Macromol., 2013, 54, 37–43 CrossRef CAS PubMed.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- D. Shugar, The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme, Biochim. Biophys. Acta, 1952, 8, 302–309 CrossRef CAS.
- Q. Wang, X. Fan, Y. J. Hu, L. Cui and P. Wang, Antibacterial functionalization of wool fabric via immobilizing lysozyme, Bioprocess Biosyst. Eng., 2009, 32, 633–639 CrossRef CAS PubMed.
- C. Huang, D. H. Zhu, H. Wu, W. Y. Lou and M. H. Zong, Evaluating the influence of inhibitors present in lignocellulosic hydrolysates on the cell membrane integrity of oleaginous yeast Trichosporon fermentans by flow cytometry, Process Biochem., 2014, 49, 395–401 CrossRef CAS PubMed.
- V. Babu, S. K. Pasha, G. Gupta, C. B. Majumdar and B. Choudhury, Enzymatic Surface Modification of Polyacrylonitrile and its Copolymers: Effects of Polymer Surface Area and Protein Adsorption, Fibers Polym., 2014, 15, 24–29 CrossRef CAS.
- X. L. Zhang, C. F. Xiao, X. Y. Hu and Z. Y. Zhang, Characterization of membranes prepared from PVDF/PAN blends and their modification with hydrolysis, High Perform. Polym., 2012, 25, 165–173 CrossRef PubMed.
- B. Zhou, R. Li, H. B. Deng, Y. Hu and B. Li, Antibacterial multilayer films fabricated by layer-by-layer immobilizing lysozyme and gold nanoparticles on nanofibers, Colloids Surf., B, 2014, 116, 432–438 CrossRef CAS PubMed.
- S. Wang, H. Fang, W. Yk, M. H. Cai, W. Liu, S. B. He and X. P. Xu, Applications of HRP-immobilized catalytic beads to the removal of 2,4-dichlorophenol from wastewater, RSC Adv., 2015, 5, 57286–57292 RSC.
- I. Kim, H. G. Yun and K. H. Lee, Preparation of asymmetric polyacrylonitrile membrane with small pore size by phase inversion and post-treatment process, J. Membr. Sci., 2002, 199, 75–84 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |