The bisindolylmaleimides with anti-parallel conformation by N-dodecyl chains on indole rings: thermal property and intensive solid-state fluorescence in single crystal†
Abstract
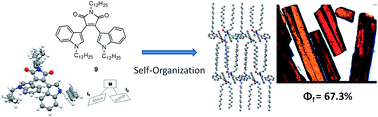
Red emitting materials containing D(donor)–A(acceptor) systems with “pyramid-mimetic” structures often gave intensive red fluorescence in the solid state. However, there is no guiding approach to fix such conformations of bisindolylmaleimides (BIMs) in the solid state. In this paper, the location of N-dodecyl chains on indole rings of BIMs was found to have the “pyramid-mimetic” structure and thus gave intensive solid-state red fluorescence in single crystals.

 Please wait while we load your content...
Please wait while we load your content...