Rationally designed hierarchical ZnCo2O4/polypyrrole nanostructures for high-performance supercapacitor electrodes†
Tingting Chen ab,
Yong Fan*a,
Guangning Wangab,
Qing Yangb and
Ruixiao Yanga
ab,
Yong Fan*a,
Guangning Wangab,
Qing Yangb and
Ruixiao Yanga
aThe College of Material Science and Engineering, Harbin University of Science and Technology, Harbin, 150040, P. R. China. E-mail: fyzf318@163.com
bThe Key Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education, School of Physics and Electronic Engineering, Harbin Normal University, Harbin, 150025, P. R. China
First published on 25th August 2015
Abstract
Electrodes with rationally designed hybrid nanostructures can offer many opportunities for enhanced performance in electrochemical energy storage. In this work, hierarchical ZnCo2O4/polypyrrole (PPy) nanostructures on Ni foam were rationally designed and successfully fabricated through a facile two-step method and were directly used as an integrated electrode for supercapacitors. The novel nanoscale morphology has been proven to be responsible for their excellent capacitive performance. When used as electrodes in supercapacitors, the hybrid nanostructures demonstrated prominent electrochemical performance with a high specific capacitance (1559 F g−1 at a current density of 2 mA cm−2), a good rate capability (89% when the current density increases from 2 to 20 mA cm−2), and a good cycling ability (90% of the initial specific capacitance remained after 5000 cycles at a high current density of 10 mA cm−2). Moreover, the high specific energy density is 30.9 W h kg−1 at a current density of 2 mA cm−2 in a two-electrode system. The excellent electrochemical performance of hierarchical ZnCo2O4/PPy nanostructures can be mainly ascribed to the enhanced adherent force between electrode materials and Ni foam to hold the electrode fragments together by means of ZnCo2O4 nanowires, the good electrical conductivity of PPy, and the short ion diffusion pathway in ordered porous PPy nanofilms and ZnCo2O4 nanowires.
Introduction
In recent years, owing to increasing concern about the rapid depletion of fossil fuels and increasingly worsening environmental pollution, energy storage is becoming one of the great challenges to maintain a healthy, prompt and sustainable economic development. As novel devices for energy storage, supercapacitors are distinguished from other types of electrochemical energy storage by their advantages of short charging times, good reversibility, high power density, and long cycle life, which have attracted increasing attention in the past few decades.1,2 Supercapacitors can be divided into electrical double layer capacitors and pseudocapacitors (PCs) according to the charge storage mechanisms.3 In particularly, capacitance of PCs arises from the fast reversible faradic redox reactions at the electrode surface, thus PCs usually provide much higher specific capacitance than supercapacitors made of carbonaceous materials based on electric double-layer charge storage.4,5 Thus, a number of current studies have been focused on PCs.Since the first report of electrical conductivity in a conjugated polymer in 1977 was presented by H. Shirakawa et al.,6 conducting polymers have received much attention because they combine the electrical properties of metals and the advantages of polymers.7 Among the conducting polymers for the PCs, polypyrrole (PPy) is one of the most promising conducting polymers for supercapacitors8,9 because of its high conductivity (10–100 S m−1),10 facile preparation, good thermal and environmental stability, environment friendly, flexibility, high redox and capacitive current.11 However, it has a relatively poor cycling stability and rate capability, because large volumetric swelling and shrinking during charge/discharge process as a result of ion doping and dedoping.12 This volumetric alternation often leads to structural breakdown and thus fast capacitance decay of conducting polymers.13,14 Apparently, most PPy-based electrode retain less than 50% of the initial capacitance after cycling for 1000 times. PPy/carbon prepared by An et al.11 presented high specific capacitance (433 F g−1 at 1 mV s−1), but poor cycling stability 36% after 2000 cycles at 1 A g−1. Therefore, cycling instability and rate capability is a major obstacle for practical applications of conductive polymer electrodes. Recently, much effort has been focused on the development of hybrid nanomaterials based on PPy in order to improve the stability, such as metal–polypyrrole core–shell nanoparticles and metal–polypyrrole hybrid nanowires.15–18 In these hybrid materials, metal nanoparticles functions as a skeleton for docking electroactive materials that can hold the electrode fragments together, and improve the stability of PPy. Particularly, ZnCo2O4 exhibits a higher stability than that of PPy materials.19–24 ZnCo2O4 nanowire prepared by Li et al.25 presented excellent cycling stability 90% after 6000 cycles at 20 mA cm−2. In this sense, nanostructured ZnCo2O4 is considered to be an excellent skeleton for docking electroactive materials. For example, Chuo et al.26 take full advantage of ZnCo2O4 to enhance the adhere force between electrodes and Ni foam to improve the stability of Ni(OH)2, in which the ZnCo2O4 nanowires act as the backbone and provide “superhighway” for electron, in addition, the ZnCo2O4 nanowires can solidify electrode materials to Ni foam and thus avoids electrode materials to fall off. These works imply that it might be possible to fabricate the hybrid electrode of ZnCo2O4/PPy with excellent electrochemical performance.
Based on the considerations above, in this work, we have designed porous hierarchical ZnCo2O4/PPy hybrid nanostructures on Ni foam. This electrode design has many apparent advantages such as: (1) the highly porous ZnCo2O4 nanowires directly grown on conductive Ni foam can enhance the adhere force between electrode materials and substrate to hold the electrode fragments together, and improve the stability of PPy. (2) The high electrical conductive PPy, which has the smaller internal resistances and diffusion resistance, led to enhanced electrochemical activity of ZnCo2O4/PPy hybrid materials. Intimately covered PPy increases both the electrical conductivity of each ZnCo2O4 nanowire and the electrical contact of ZnCo2O4 with nickel foam current collector, leading to the maximum utilization of ZnCo2O4 during the electrochemical reactions. (3) The porous ZnCo2O4 nanowires and porous PPy nanofilms growing onto the ZnCo2O4 nanowires have a high surface area and provide more active reaction sites, improving the utilization of active materials not only on the active material surface but also throughout the bulk. (4) The wire structure and the large open space between the nanofilms can greatly enhance the kinetics of ion and electron transport inside the electrodes. The abundant space between individual nanostructures allows for facile diffusion of electrolyte into inner region of the electrode.
Experiments
In the experiment, all the reagents were of analytical grade and used without any further purification.Synthesis of ZnCo2O4 nanowires: before the fabrication of ZnCo2O4 samples, the nickel foam substrate was treated with 6 M HCl in an ultrasound for 20 min to remove the oxide layer, and then rinsed with deionized water for 30 min. In a typical synthesis, Zn(NO3)2·6H2O (1 mmol, 0.297 g), Co(NO3)2·6H2O (2 mmol, 0.582 g), CO(NH2)2 (5 mmol, 0.300 g) and NH4F (2 mmol, 0.074 g) were added together into 40 mL deionized water under stirring, forming a clear red solution. Sequentially, the mixed solution and a piece of pretreated Ni foam were transferred together into a capacity of 50 mL Teflon-lined stainless steel autoclave, heated in an oven at 120 °C for 5 h, and then cooled down to ambient temperature naturally. The Ni foam coated with a pink product was taken out from the autoclave and rinsed carefully with deionized water, then annealed in an oven at 400 °C for 2 h.
Synthesis of hierarchical ZnCo2O4/PPy nanostructures: the hybrid nanostructures were obtained by electrodeposition with a Lab-logic electrochemical workstation (VMP3, France). Primarily, ZnCo2O4 nanowires was immersed in pyrrole/acetone solution with a concentration of 0.5 M for 0.5 h. Then, the ZnCo2O4 nanowires with pyrrole (Py) monomers adsorbed on the surface of each individual ZnCo2O4 nanowires was directly used as the working electrode under a potential of 0.8 V in 0.3 M LiClO4 aqueous solution. A Pt wire and Ag/AgCl were used as the counter and reference electrodes, respectively. A typical electropolymerization time was 5 min. After the electropolymerization process, the hierarchical ZnCo2O4/PPy nanostructures were obtained. The nickel foam supported hybrid nanostructure (∼1.5 cm2 area; ZnCo2O4 mass: 1.426 mg; PPy mass: 1.434 mg) or pristine ZnCo2O4 nanowires acted directly as the working electrode. Pure PPy were deposited on Ni foam for 5 minutes in order to have the same thickness of PPy with the hierarchical ZnCo2O4/PPy nanostructures.
The sample morphology and structure of the nanocomposites were characterized by scanning electron microscopy (SEM, Hitachi SU 70), transmission electron microscopy (TEM; FEI, Tecnai TF 20), Fourier transform infrared (FTIR, BRUKER VERTEX 80) and X-ray diffraction (XRD; D/max-2600/PC, Rigaku, Japan) with Cu Kα radiation (λ = 1.5418 Å) in the 2θ of 20–70°.
The electrochemical properties were measured on a Lab-logic electrochemical workstation (VMP3, France) using a three-electrode configuration in 3 M KOH, where the ZnCo2O4/PPy nanostructure served as working electrode, a platinum plate and Ag/AgCl as the counter and reference electrodes, respectively. Cyclic voltammetry (CV) tests were recorded from −0.1 to 0.58 V at scan rates of 5, 15, 25, and 40 mV s−1 at room temperature. The galvanostatic charge/discharge (GCD) property was measured at the current densities of 2, 5, 10, 15, and 20 mA cm−2 with the potential in range of 0 to 0.44 V. Electrochemical impedance spectroscopy (EIS) measurements were carried out on this apparatus in the frequency range of 100 kHz to 10 mHz. The specific capacitance (Cs), area capacitance (Ca) was calculated using the following equation:
 | (1) |
 | (2) |
In a two-electrode system, the specific energy (E) and the specific power (P) was calculated using the following equation:
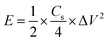 | (3) |
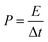 | (4) |
Results and discussion
The representative synthetic procedure of the hybrid nanostructures electrode is illustrated in Fig. 1. First, commercial nickel foam was employed as the current collector to grow ZnCo2O4 nanowire via a modified hydrothermal and annealing process. Then, the ZnCo2O4 nanowire was immersed into a Py solution to adsorb a layer of Py molecules on the nanowire surface. Finally, the Py monomers were crosslinked into a solid PPy layer by electropolymerization in a three-electrode cell with the sponge as the working electrode. Fig. 2a shows typical SEM images of the as-synthesized ZnCo2O4 nanowires on Ni foam. Fig. 2b is the enlarged SEM image of a local area in the Fig. 2a. From the magnified SEM image, it can be seen, that the nanowires have an average diameter of 40 nm and the length is about 7 μm. The further magnified view in the Fig. 2c shows the porous structure of ZnCo2O4 nanowires, which can be due to the release of gas during the calcining process.27,28 Fig. 2d demonstrates a representative low-magnification SEM image of the hierarchical ZnCo2O4/PPy nanostructures. Fig. 2e shows a magnified SEM image of the local area in the Fig. 2d. Obviously, the ZnCo2O4 nanowires uniformly pass through the PPy nanofilms to form nanoarray network. From the magnified SEM image in the Fig. 2f, it can be found that porous PPy nanofilms were deposited onto the surface of ZnCo2O4 nanowires. The porous nanostructure of PPy was formed by the oxidation and reduction process of the polymer during its growth progress.29 Such a unique nanoarray network nanostructure has open and free interspaces among themselves (Fig. S1a†), which assures that electrolytes are highly accessible to all the electrode materials and then could improve the utilization rate of electrode materials, thus providing more surface area for easy diffusion of the electrolyte into the inner interface of the electrode materials. | ||
| Fig. 1 The representative synthetic procedure and structure details of the hybrid nanostructure electrode. | ||
 | ||
| Fig. 2 (a) Low and (b), (c) high-magnification SEM images of ZnCo2O4 nanowires. (d) Low and (e), (f) high-magnification SEM images of the hierarchical ZnCo2O4/PPy nanostructures. | ||
The microstructures of the ZnCo2O4 and ZnCo2O4/PPy hybrid nanostructures were further investigated using TEM. Fig. 3a shows a TEM image of a single ZnCo2O4 nanowire with a diameter of ∼40 nm that further confirms the presence of the highly porous structure, which is consistent with SEM observation. Fig. 3b shows a HRTEM image of the porous ZnCo2O4 nanowire. It reveals an interplanar spacing of 0.29 nm and 0.25 nm, corresponding to the (220) and (311) lattice planes of ZnCo2O4. The typical TEM image of the hierarchical ZnCo2O4/PPy nanostructure is shown in Fig. 3c. It is evidently observed that the ZnCo2O4 is shelled by an amorphous PPy layer, forming a typical core–shell structure. The HRTEM examination shown in Fig. 3d reveals the amorphous PPy layer with the thickness of more than ten nanometers. In addition, some PPy nanofilms are attached tightly to the nanowire in the particulate form with visibly larger sizes, as shown in Fig. S1b.† TEM image of the hierarchical ZnCo2O4/PPy nanostructure shows that PPy is either immobilized onto the surface of ZnCo2O4 nanowires as a nanosized thin layer or firmly attached to the nanowires in the form of nanofilms, and the two kinds of existing PPy in the hybrid structure would definitely facilitate the electron transport and enhance the electrical connection with the current collector.30 These features enable our hybrid electrode to satisfy the critical requirements for electrochemical capacitors. Moreover, it is clearly shown in Fig. S2† that the elements N, C, O, Co, and Zn are homogeneously distributed, achieving the uniform coating of PPy on the ZnCo2O4 NWs.
The Fourier transform infrared spectroscopy (FTIR) spectrum provides further evidence for the presence of PPy (Fig. 3e). In detail, the first broad band located at 3346 cm−1 corresponds to N–H bonds of aminegroups.30 The two peaks at 2968 and 2872 cm−1 correspond to aliphatic C–H groups.31 The peak observed at 2357 cm−1 is associated with the hydroxyl vibration.32 The peak at 1408 cm−1 is due to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H in-plane vibration, the band at 1089 cm−1 is characteristic of the C–N stretching vibration, and the band at 1012 cm−1 is attributed to C–H and N–H in-plane ring deformation vibration.33–35 The slight shift of the FTIR peaks of PPy is considered to arise from the interfacial interaction between PPy and ZnCo2O4. A typical XRD pattern of the ZnCo2O4 and ZnCo2O4/PPy hybrid nanostructures are shown in Fig. 3f. The diffraction peaks of pure ZnCo2O4 can unambiguously demonstrate the structure of face-centered cubic ZnCo2O4 (JCPDS card no. 23-1390). It can be observed that the XRD pattern of ZnCo2O4/PPy hybrid nanostructures has a similar XRD pattern of ZnCo2O4. However, different from the peak of ZnCo2O4, the peak of ZnCo2O4/PPy hybrid nanostructures exhibits another broad reflection located in the range of 20–30°, which is attributed to the diffraction patterns of amorphous PPy.36 On the basis of the characterizations above, it can be confirmed that the PPy was successfully coated on the surface of the nanowires.
C–H in-plane vibration, the band at 1089 cm−1 is characteristic of the C–N stretching vibration, and the band at 1012 cm−1 is attributed to C–H and N–H in-plane ring deformation vibration.33–35 The slight shift of the FTIR peaks of PPy is considered to arise from the interfacial interaction between PPy and ZnCo2O4. A typical XRD pattern of the ZnCo2O4 and ZnCo2O4/PPy hybrid nanostructures are shown in Fig. 3f. The diffraction peaks of pure ZnCo2O4 can unambiguously demonstrate the structure of face-centered cubic ZnCo2O4 (JCPDS card no. 23-1390). It can be observed that the XRD pattern of ZnCo2O4/PPy hybrid nanostructures has a similar XRD pattern of ZnCo2O4. However, different from the peak of ZnCo2O4, the peak of ZnCo2O4/PPy hybrid nanostructures exhibits another broad reflection located in the range of 20–30°, which is attributed to the diffraction patterns of amorphous PPy.36 On the basis of the characterizations above, it can be confirmed that the PPy was successfully coated on the surface of the nanowires.
In order to explore the electrochemical properties of ZnCo2O4/PPy hybrid nanostructures, both CV and GCD cycling measurement were carried out with 3 M KOH aqueous solution in a three-electrode system. As shown in Fig. 4a, the enclosed area of the ZnCo2O4/PPy hybrid electrode is obviously larger than that of ZnCo2O4 and PPy. This increased area is mainly contributed by synergistic effects36 between ZnCo2O4 and PPy. The CV and GCD measurements of the PPy and ZnCo2O4 electrodes were performed at various scan rates and various current densities to compare the electrochemical activities (Fig. S3†). From the obtained GCD profiles of the three electrodes as shown in Fig. S4,† the Cs of the hybrid electrodes, ZnCo2O4 nanowire electrodes and PPy electrodes are calculated to be 1559 F g−1, 1275 F g−1, and 180 F g−1, respectively. The value of ZnCo2O4/PPy hybrid electrode is higher than that of ZnCo2O4 and PPy. The larger Cs for ZnCo2O4/PPy is due to the improvement of the electronic conductivity between the particles, owing to their PPy coat. Fig. 4b shows the CV curves of the ZnCo2O4/PPy hybrid nanostructures supported on Ni foam at various scanning rates ranging from 5 to 40 mV s−1. Obviously, a pair of distinct redox peaks are visible in the CV curves with the potential windows ranging from −0.1 to 0.58 V, demonstrating that the capacitance characteristics are mainly ascribed to the faradic capacitive behavior. Interestingly, the redox current almost linearly increases with increasing scan rate, demonstrating that the kinetics of interfacial faradic redox reactions and the rate of electronic and ionic transport are rapid enough.37 And also, the reduction and oxidation peaks slightly shift toward lower and higher potential, respectively, which can be attributed to the polarization effect of the electrode.38
To estimate the specific capacitance of the ZnCo2O4/PPy as supercapacitor electrodes, GCD measurements were further performed at various current densities of 2, 5, 10, 15 and 20 mA cm−2. As shown in Fig. 4c, the potential plateaus in the discharge curves match well with the reduction peaks observed in the CV curves, which correspond to the reductive process, and this phenomenon indicates good pseudocapacitive behaviors. The Cs of PPy, ZnCo2O4 and ZnCo2O4/PPy hybrid electrodes at various current densities are shown in Fig. 4d. The corresponding calculated values are provided in ESI.† The Cs measured in the same way is reported in Table S1.† The Cs of the ZnCo2O4/PPy hybrid electrodes are calculated to be 1559, 1500, 1442, 1414, and 1388 F g−1 at current densities of 2, 5, 10, 15, and 20 mA cm−2, respectively, according to formula (1). The Cs decreases when the current density increases, which reflects the loss of efficiency of the active material at high current densities. The corresponding Ca is 2.54, 2.50, 2.43, 2.39, and 2.35 F cm−2, respectively, according to formula (2). Most importantly, the Cs of the ZnCo2O4/PPy hybrid electrodes still keeps at 1388 F g−1 at a high density of 20 mA cm−2, 89% of that at 2 mA cm−2, highlighting the excellent rate capability of the ZnCo2O4/PPy hybrid electrodes. It can be noted that the capacitance retention is 53.3%, 73.8%, and 89% as the current density is increased from 2 to 20 mA cm−2 for PPy, ZnCo2O4 and ZnCo2O4/PPy, respectively. The rate capability of the ZnCo2O4/PPy electrodes is better than that of PPy and ZnCo2O4, as well as many other pseudocapacitor electrodes such as PPy@C39 (19.5% areal capacitance retention from 2 mA cm−2 to 10 mA cm−2), activated carbon/PPy40 (71.4% capacitance retention from 2 mA cm−2 to 20 mA cm−2), V2O5/PPy nanostructures41 (about 73% capacitance retention from 2 A g−1 to 10 A g−1), ZnCo2O4 nanorods42 (83% capacitance retention from 2 A g−1 to 20 A g−1).
The durability of the electrode materials is a critical aspect for supercapacitor applications. The cycling performance was conducted in the potential windows ranging from 0 to 0.44 V. Fig. 4e shows the cycling performance of 5000 cycles at a current density of 10 mA cm−2 of PPy, ZnCo2O4 and ZnCo2O4/PPy. During the first 1400 cycles at 10 mA cm−2, the Cs was increasing, and then decreasing during the last 3600 cycles and finally maintained at about 90% after 5000 cycles. The increasing Cs can be mainly attributed to the activation process of the active materials in the electrolyte.43 With the cycle number increasing, the ions of electrolyte first go through PPy, and then diffuse to the inter ZnCo2O4 absolutely and opened up the ion access. The pure PPy electrodes exhibit 25% capacitance retention. The ZnCo2O4/PPy composite electrodes display 90% capacitance retention after 5000 cycles, which is quite superior to that of PPy nanofilms and is better than the reported cycling stability of PPy/MnO2 (about 54% after 500 cycles),44 V2O5–PPy (80% after 5000 cycles),45 and ZnO/PPy (88% after 5000 cycles),46 Fig. 4e inset shows the 1st and the 5000st GCD curves of charge–discharge cycling tests at the scan rate of 2 mA cm−2. After 5000 cycles, the shape of curve almost unchanged compared with the 1st cycling curve, indicating stable electrochemical properties of the active material. Such superior cycling stability of hierarchical ZnCo2O4/PPy nanostructures can be mainly ascribed to the enhanced adherent force between electrode materials and Ni foam by means of ZnCo2O4 nanowires,26,47 the electrode fragments held together, and the improved stability of PPy. These results indicate excellent electrochemical stability and good reversible redox behaviors, which have huge application potential in supercapacitors.
Fig. 4f shows the Nyquist plots of the EIS spectra for the PPy, ZnCo2O4, and ZnCo2O4/PPy hybrid electrodes and the inset shows expanded view in the high- and mid-frequency region. At the high frequency, the intersection of the curve at the real part indicates the resistance of the electrochemical system (Rs, which includes the inherent resistance of the electroactive material, ionic resistance of electrolyte, and contact resistance at the interface between electrolyte and electrode). From this picture, the Rs for ZnCo2O4/PPy is smaller than others (which was measured to be 0.31, 0.37, and 0.30 Ω for PPy, ZnCo2O4 and ZnCo2O4/PPy respectively.) The charge & transfer resistance (Rct) of the ZnCo2O4/PPy is obviously smaller than that of the bare ZnCo2O4 and PPy, suggesting that the hybrid electrode is beneficial to accelerate the charge transfer. In the low frequency area, the slope of the curve shows the Warburg resistance (Zw), which represents the electrolyte diffusion to the electrode surface.48 A more ideal straight line (a larger straight-line slope arises from low frequency) can be observed for ZnCo2O4/PPy than that for ZnCo2O4 and PPy, indicating the improved capacitive properties for the hybrid.49,50 By contrast, ZnCo2O4/PPy displays smallest Rct in addition to the small Rs, indicating the best electrical conductivity and electroactivity.
The two-electrode supercapacitor geometry has been suggested to be the best indication of an electrode material's performance.51 Fig. 5a shows the CV curves of the hierarchical ZnCo2O4/PPy nanostructures at various scanning rates ranging from 5–100 mV s−1 in a potential window of −0.8 V to +0.8 V. The reduction and oxidation peaks on CV curves can be observed and the peak current almost linearly increases with increasing scan rate, in agreement with the trend revealed by the three-electrode system. GCD measurements were further performed at various current densities of 2, 5, 10, 15 and 20 mA cm−2 and the resultant profiles are given in Fig. 5b. The CV curves and GCD curves of PPy and ZnCo2O4 were also measured in two-electrode system for comparison (Fig. S5†). Specific energy and specific power are two key factors to evaluate the power applications of electrochemical supercapacitors, which were calculated from the discharge curves according to the eqn (3) and (4), respectively. The energy density and power density performance of PPy, ZnCo2O4, and ZnCo2O4/PPy electrodes are shown in Fig. 5c. Obviously, the ZnCo2O4/PPy hybrid electrodes have the best energy density and power density performance. As seen from the Ragone plots, as the power density increases from 0.5 kW kg−1 to 5.8 kW kg−1 when the current density increases from 2 to 20 mA cm−2, while the energy density decreases from 30.9 to 27.5 W h kg−1. These values are remarkable compared to the most reported electrode materials, such as ZnCo2O4 electrode52 with energy density of 12.5 W h kg−1 when power density is 0.8 kW kg−1 at 1.2 mA cm−2, PPy@MoO3//AC(+) device53 with an energy density of 20 W h kg−1 when power density is 0.75 kW kg−1, CNT@PPy@MnO2 electrode54 with the maximum energy density and power density are 8.6 W h kg−1 and 16.5 kW kg−1. The results demonstrate that the ZnCo2O4/PPy hybrid materials have excellent electrochemical properties of high energy density and power output in hybrid vehicle systems.
The excellent electrochemical performance can be due to following reasons: (1) the dramatic performance improvement after PPy integration can first be attributed to the smaller Rs (0.30 vs. 0.37 Ω) and diffusion resistance of the ZnCo2O4/PPy hybrid electrode as compared to the pure ZnCo2O4 electrode in the Fig. 5f. Intimately covered PPy increases both the electrical conductivity of each ZnCo2O4 nanowire and the electrical contact of ZnCo2O4 with nickel foam current collector, leading to the maximum utilization of ZnCo2O4 during the electrochemical reactions.30 (2) Secondly, we believe that ZnCo2O4 nanowires can in reverse serve as efficient scaffold to ensure the fine distribution of PPy at nanoscale, making them fully electrochemically accessible, and enhance adherent force between electrode materials and Ni foam to hold the PPy electrode fragments together. (3) In addition, the porous structure of nanowires and nanofilms not only provided more electroactive sites for the faradic reactions, but also shortened the distance of OH ion/electron transformation, which led to faster kinetics and enhanced electrochemical performance.55 (4) ZnCo2O4 nanowires and PPy nanofilms were connected with each other to form a nanoarray network, thus the void space can buffer the volume change during the GCD processes, which has led to enhanced stability of ZnCo2O4/PPy nanostructures, and the contact resistance of the whole electrode could be reduced. Secondly, staggered and separated growth of nanofilms not only reduced the free space on the surface of Ni substrate but also increased the effective contact area between electrode and electrolyte, which significantly improved the utilization of active materials.55 Thus, the synergetic contribution from the two promising pseudocapacitive materials (ZnCo2O4 and PPy) together with the merits of porous nanoarray network architecture56 should account for the exceptionally high capacitance and better rate capability of the ZnCo2O4/PPy hybrid electrode, which are encouraging since our electrode is entirely carbon additive- and binder-free.
Conclusions
ZnCo2O4/PPy hybrid electrodes are successfully synthesized on Ni foam. They exhibited a prominent electrochemical performances of 1559 F g−1 at 2 mA cm−2 and a high energy density of 30.9 W h kg−1 at a power density of 0.2 kW kg−1. After charging/discharging for 5000 cycles at a high current density of 10 mA cm−2, 90% of the initial specific capacitance was retained. Such promising electrochemical stability mainly attributed to the ZnCo2O4 nanowires that enhanced adherent force between electrode materials and Ni foam and hindered the structural degradation of the polymer. In addition, good electrical conductivity rendered by PPy integration, in combination with facile ion diffusion path provided by both the porous structures of PPy nanofilms and ZnCo2O4 nanowires, give rise to enhanced rate capability. Our work opens up the possibility to engineer ZnCo2O4/PPy hybrid nanostructures into a promising pseudocapacitive material.Acknowledgements
This work was partially supported by the Natural Science Foundation of China (No. 51277044).References
- A. Rudge, J. Davey, I. Raistrick, S. Gottesfeld and J. P. Ferraris, J. Power Sources, 1994, 47, 89 CrossRef CAS
.
- X. Peng, L. Peng, C. Wu and Y. Xie, Chem. Soc. Rev., 2014, 43, 3303 RSC
.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS
.
- L. Huang, D. C. Chen, Y. Ding, S. Feng, Z. L. Wang and M. L. Liu, Nano Lett., 2013, 13, 3135 CrossRef CAS PubMed
.
- X. Y. Cao, X. Xing, N. Zhang, H. Gao, M. Y. Zhang, Y. C. Shang and X. T. Zhang, J. Mater. Chem. A, 2015, 3, 3785 CAS
.
- H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc., Chem. Commun., 1977, 578 RSC
.
- A. K. Bakhshi and G. Bhalla, J. Sci. Ind. Res., 2004, 63, 715 CAS
.
- K. Jurewicz, S. Delpeux, V. Bertagna, F. Beguin and E. Frackowiak, Chem. Phys. Lett., 2001, 347, 36 CrossRef CAS
.
- T. E. Campbell, A. J. Hodgson and G. G. Wallace, Electroanalysis, 1999, 11, 215 CrossRef CAS
.
- S. Kuwabata, J. Nakamura and H. Yoneyama, J. Electrochem. Soc., 1990, 137, 2147 CrossRef CAS PubMed
.
- H. F. An, Y. Wang, X. Y. Wang, L. P. Zheng, X. Y. Wang, L. H. Yi, L. Bai and X. Y. Zhang, J. Power Sources, 2010, 195, 6964 CrossRef CAS PubMed
.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025 CrossRef CAS PubMed
.
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976 CrossRef CAS PubMed
.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC
.
- S. T. Selvan, J. P. Spatz, H. A. Klok and M. Moller, Adv. Mater., 1998, 10, 132 CrossRef CAS
.
- L. Qiu, Y. Peng, B. Liu, B. Lin, Y. Peng, M. J. Malik and F. Yan, Appl. Catal., A, 2012, 230, 413 Search PubMed
.
- W. K. Chee, H. N. Lim, I. Harrison, K. F. Chong, Z. Zainal, C. H. Ng and N. M. Huang, Electrochim. Acta, 2015, 157, 88 CrossRef CAS PubMed
.
- V. Branzoi, F. Branzoi and L. Pilan, Mater. Chem. Phys., 2009, 118, 197 CrossRef CAS PubMed
.
- M. Davis, C. Guemeci, B. Black, C. Korzeniewski and L. Hope-Weeks, RSC Adv., 2012, 2, 2061 RSC
.
- X. Song, Q. Ru, B. B. Zhang, S. J. Hu and B. N. An, J. Alloys Compd., 2014, 585, 518 CrossRef CAS PubMed
.
- G. Zhou, J. Zhu, Y. J. Chen, L. Mei, X. C. Duan, G. H. Zhang, L. B. Chen, T. H. Wang and B. G. Lu, Electrochim. Acta, 2014, 123, 450 CrossRef CAS PubMed
.
- H. Wu, Z. Lou, H. Yang and G. Z. Shen, Nanoscale, 2015, 7, 1921 RSC
.
- S. Chen, M. Xue, Y. Q. Li, Y. Pan, L. K. Zhu, D. L. Zhang, Q. R. Fang and S. L. Qiu, Inorg. Chem. Front., 2015, 2, 177 RSC
.
- B. Liu, B. Y. Liu, Q. F. Wang, X. F. Wang, Q. Y. Xiang, D. Chen and G. Z. Shen, ACS Appl. Mater. Interfaces, 2013, 5, 10011 CAS
.
- B. K. Guan, D. Guo, L. L. Hu, G. H. Zhang, T. Fu, W. J. Ren, J. D. Li and Q. H. Li, J. Mater. Chem. A, 2014, 2, 16116 CAS
.
- H. X. Chuo, H. Gao, Q. Yang, N. Zhang, W. B. Bu and X. T. Zhang, J. Mater. Chem. A, 2014, 2, 20462 CAS
.
- S. B. Wang, J. Pu, Y. Tong, Y. Y. Chen, Y. Gao and Z. H. Wang, J. Mater. Chem. A, 2014, 2, 5434 CAS
.
- Q. H. Wang, L. X. Zhu, L. Q. Sun, Y. C. Liu and L. F. Jiao, J. Mater. Chem. A, 2015, 3, 982 CAS
.
- L. Z. Fan and J. Maier, Electrochem. Commun., 2006, 8, 937 CrossRef CAS PubMed
.
- G. J. Cruz, M. G. Olayo, O. G. López, L. M. Gómez, J. Morales and R. Olayo, Polymer, 2010, 51, 4314 CrossRef CAS PubMed
.
- P. Heyse, R. Dams, S. Paulussen, K. Houthoofd, K. Janssen and P. A. Jacobs, et al., Plasma Processes Polym., 2007, 4, 145 CrossRef CAS PubMed
.
- C. Zhou, Y. W. Zhang, Y. Y. Li and J. P. Liu, Nano Lett., 2013, 13, 2078 CrossRef CAS PubMed
.
- B. B. Yue, C. Y. Wang, X. Ding and G. G. Wallace, Electrochim. Acta, 2012, 68, 18 CrossRef CAS PubMed
.
- D. Zhang, Q. Q. Dong, X. Wang, W. Yan, W. Deng and L. Y. Shi, J. Phys. Chem. C, 2013, 117, 20446 CAS
.
- X. Zhang, W. S. Yang and Y. W. Ma, Electrochem. Solid-State Lett., 2009, 12, A95 CrossRef CAS PubMed
.
- G. F. Ma, H. Peng, J. J. Mu, H. H. Huang, X. Z. Zhou and Z. Q. Lei, J. Power Sources, 2013, 229, 72 CrossRef CAS PubMed
.
- X. J. Ma, L. B. Kong, W. B. Zhang, M. C. Liu, Y. C. Luo and L. Kang, RSC Adv., 2014, 4, 17884 RSC
.
- D. P. Cai, D. D. Wang, B. Liu, L. L. Wang, Y. Liu, H. Li, Y. R. Wang, Q. H. Li and T. H. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 5050 CAS
.
- T. Y. Liu, L. Finn, M. H. Yu, H. Y. Wang, T. Zhai, X. H. Lu, Y. X. Tong and Y. Li, Nano Lett., 2014, 14, 2522 CrossRef CAS PubMed
.
- B. Muthulakshmi, D. Kalpana, S. Pitchumani and N. G. Renganathan, J. Power Sources, 2006, 158, 1533 CrossRef CAS PubMed
.
- T. Qian, N. Xu, J. Q. Zhou, T. Z. Yang, X. J. Liu, X. W. Shen, J. Q. Liang and C. L. Yan, J. Mater. Chem. A, 2015, 3, 488 CAS
.
- B. Liu, B. Y. Liu, Q. F. Wang, X. F. Wang, Q. Y. Xiang, D. Chen and G. Z. Shen, ACS Appl. Mater. Interfaces, 2013, 5, 10011 CAS
.
- B. K. Guan, D. Guo, L. L. Hu, G. H. Zhang, T. Fu, W. J. Ren, J. D. Li and Q. H. Li, J. Mater. Chem. A, 2014, 2, 16116 CAS
.
- A. Bahloul, B. Nessark, E. Briot, H. Groult, A. Mauger, K. Zaghib and C. M. Julien, J. Power Sources, 2013, 240, 267 CrossRef CAS PubMed
.
- M. H. Bai, L. J. Bian, Y. Song and X. X. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 12656 CAS
.
- N. K. Sidhu and A. C. Rastogi, Nanoscale Res. Lett., 2014, 9, 453 CrossRef PubMed
.
- G. W. Yang, C. L. Xu and H. L. Li, Chem. Commun., 2008, 6537 RSC
.
- X. Y. Liu, S. J. Shi, Q. Q. Xiong, L. Li, Y. J. Zhang, H. Tang, C. D. Gu, X. L. Wang and J. P. Tu, ACS Appl. Mater. Interfaces, 2013, 5, 8790 CAS
.
- A. Davies, P. Audette, B. Farrow, F. Hassan, Z. Chen, J. Y. Choi and A. Yu, J. Phys. Chem. C, 2011, 115, 17612 CAS
.
- H. H. Chang, C. K. Chang, Y. C. Tsai and C. S. Liao, Carbon, 2012, 50, 2331 CrossRef CAS PubMed
.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 CAS
.
- S. B. Wang, J. Pu, Y. Tong, Y. Y. Chen, Y. Gao and Z. H. Wang, J. Mater. Chem. A, 2014, 2, 5434 CAS
.
- Y. Liu, B. Zhang, Y. Yang, Z. Chang, Z. Wen and Y. Wu, J. Mater. Chem. A, 2013, 1, 13582 CAS
.
- P. X. Li, Y. B. Yang, E. Z. Shi, Q. C. Shen, Y. Y. Shang, S. T. Wu, J. Q. Wei, K. L. Wang, H. W. Zhu, Q. Yuan, A. Y. Cao and D. H. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 5228 CAS
.
- F. X. Bao, X. F. Wang, X. D. Zhao, Y. Wang, Y. Ji, H. D. Zhang and X. Y. Liu, RSC Adv., 2014, 4, 2393 RSC
.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: (Fig. S1) SEM and TEM images, and (Fig. S2) mapping images of hybrid nanostructures; the CV curves and GCD curves of PPy and ZnCo2O4 (Fig. S3); GCD curves of the PPy, ZnCo2O4, and ZnCo2O4/PPy (Fig. S4); the data of Specific capacitance (Table S1); the CV curves and GCD curves of PPy and ZnCo2O4 in a two electrode system (Fig. S5). See DOI: 10.1039/c5ra14808a |
| This journal is © The Royal Society of Chemistry 2015 |



