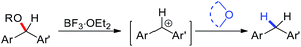
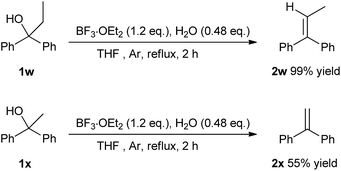
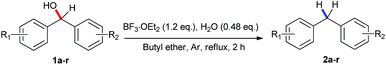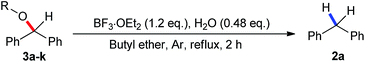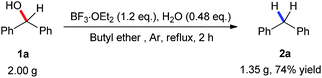Ethers as hydrogen sources in BF3·OEt2 promoted reduction of diphenylmethyl alcohols, ethers and esters to hydrocarbons†
Jiaqiang Lia,
Qing Liua,
Hang Shena,
Ruofeng Huanga,
Xiaohui Zhanga,
Yan Xiong*ab and
Changguo Chen*a
aSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China. E-mail: xiong@cqu.edu.cn; cgchen@cqu.edu.cn
bChongqing Institute of Green and Intelligent Technology, Chinese Academy of Science, Chongqing 400714, China
First published on 30th September 2015
Abstract
A novel ether/BF3 reductive system has been described, in which diphenylmethanols and their ether and ester derivatives are used as starting materials. Reductions are performed in ether under reflux and an argon atmosphere, and the addition of extra water is beneficial to this reduction. A series of alkanes are able to be prepared with good to excellent yields. A deuterated experiment exhibits that the reductive hydrogen is generated from ether. The mechanism is discussed in detail to explain the observed reactivity.
The reduction of carbon–oxygen bonds to the corresponding carbon–hydrogen bonds plays a fundamental role in organic chemistry.1,2 It is also widely applied in total synthesis chemistry, in which the C–H frame of natural products are constructed with high efficiency.3 Barton–McCombie deoxygenation is one of the most classic methods, and is well applied by transforming alcohols to their corresponding thiocarbonyl intermediates which undergo radical fragmentation.4 After Barton–McCombie deoxygenation, several alternative catalytic protocols have been developed employing metal hydrides such as organotin hydride.5,6 In these strategies, a two-step procedure firstly converts alcohols into preactivated intermediates such as thionocarbonates that are capable of being cleaved readily in reductive steps.7 Subsequently, the application of a two-step procedure is preferred in the deoxygenation of alcohol derivatives such as aryl sulfonates (e.g. triflates, tosylates, mesylates), arylthiocarbonates, ethers and esters mediated by Pd-,8 Ni-9a–c and Rh-based catalysts.9d In the past decades, many direct dehydroxylation reactions have been reported as well.10,11 Among these established methods, the combination of hydride reagents with Lewis acids such as LiAlH4/AlCl3,11a LiAlH4/TiCl3,11b and NaBH4/AlCl3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11c seems to increase the reactivity of hydride. Dehydroxylations of primary, secondary, and tertiary alcohols have been developed by employing hydrosilanes/metallic Lewis acids systems such as Ph2SiHCl/InCl3,12a Et3SiH/PdCl2
11c seems to increase the reactivity of hydride. Dehydroxylations of primary, secondary, and tertiary alcohols have been developed by employing hydrosilanes/metallic Lewis acids systems such as Ph2SiHCl/InCl3,12a Et3SiH/PdCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12b and PMHS/PdCl2.12c Reduction processes in the presence of nonmetallic Lewis acid B(C6F5)3 are also reported.13
12b and PMHS/PdCl2.12c Reduction processes in the presence of nonmetallic Lewis acid B(C6F5)3 are also reported.13
BF3·OEt2, generated from the donor–acceptor action between BF3 and Et2O,14 has shown high activity in many synthetic procedures like alkylation,15a–d cyclization,15e rearrangement,15f coupling,15g cyanation15h and etherification reactions.15i It was also utilized in the reduction of alcohols,16a,b carbonyl compounds16c and epoxides16d via hydride transfer from hydrosilanes. Our studies have demonstrated that the superacid BF3–H2O promoted benzylation reaction via a carbonium intermediate.17 We found that when iodobenzene was involved as both arene and solvent, the benzylation product was not produced and the undesired diphenylmethane was obtained in 47% yield by a reductive mechanism. The source of hydrogen was thought to come from the diphenylmethanol or diethyl ether by disproportionation, accompanied with the generation of reductive hydrogen. However, no diphenylketone was detected, which indicated that hydrogen was released very probably from the selfoxidation of ether ligand of BF3·OEt2. To test this theory, control experiments were performed in which diphenylmethanol and BF3·OEt2 in dichloromethane led to a 22% yield (see ESI†) and the later deuterated experiment resulted in the D-labelled methene.
The studies on triphenylmethyl cation, in the stable form of trityl salt, can date back to one century ago.18 Due to its importance in polymer of ether, it was regarded as an excellent initiator of polymerization and kinetically studied extensively covering the hydride ion transfer between triphenylcarbonium and ether. However, for this H transfer, there is no report on relatively unstable diphenylcarbonium ion, due to the unavailability of the salt. Herein, we would like to report our work in BF3·OEt2-promoted reductive reaction of diphenylcarbonium, using diphenylmethanols as well as their ether and ester derivatives as substrates, to corresponding alkanes, employing various ethers as hydrogen sources (Scheme 1).
Considering the hydrogen generated from the ether, we chose initially tetrahydrofuran (THF) as a solvent for the reduction of diphenylmethanol (1a), and as a result the yield was improved by up to 53% (Table 1, entry 1). As mentioned before,17 mixing BF3·OEt2 with H2O resulted in the formation of BF3–H2O which has shown elevated reactivity in the benzylation of arenes. We assessed the influence of different amounts of H2O in BF3·OEt2 promotion. 0.48 equivalent of water gave the best result while lower or higher amount of water turned out to be detrimental to this reduction (Table 1, entries 2–4). Then, the treatment of diphenylmethanol with catalytic amount (20 mol%) of BF3·OEt2 gave rise to diphenylmethane 2a in 20% yield (Table 1, entry 5). Increasing the amount of BF3·OEt2 from 0.6 to 0.8 equivalent resulted in a large improvement of the yield up to 61% (Table 1, entries 6 and 7). 1.0 equivalent of BF3·OEt2 furnished the desired product in 63% yield with the same level of reactivity compared to 1.2 equivalents of BF3·OEt2 (Table 1, entry 8 vs. 3). However, a lower yield was observed when further increasing the amount of BF3·OEt2 (Table 1, entry 9). A solvent screening with diethyl ether, and 2-methyl tetrahydrofuran was conducted and gave moderate yields (Table 1, entries 10 and 11). When methyl tert-butyl ether (MTBE) and isopropyl ether were utilized as solvent, the reactions went complex and the attempt to separate the product failed (Table 1, entries 12 and 13). To our delight, employing butyl ether as solvent exhibited excellent reactivity (Table 1, entry 14). Variations of the temperature from 100 °C to 160 °C were tested, in which an excellent yield of 87% was obtained under 160 °C (Table 1, entries 15–17). The optimal reaction conditions were established: 1.0 mmol diphenylmethanol, 1.2 equivalents of BF3·OEt2 in 1 mL of butyl ether in argon atmosphere under reflux for 2 h (Table 1, entry 17).
| Entry | BF3·OEt2 (eq.) | H2O (eq.) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Conditions: 1a (1.0 mmol), BF3·OEt2 (specified), H2O (specified) in solvent (1.0 mL) under argon and reflux (oil bath of 120 °C) for 2 h.b Isolated yields.c Complex reaction.d Oil bath of 100 °C.e Oil bath of 140 °C.f Oil bath of 160 °C. | ||||
| 1 | 1.2 | — | THF | 53 |
| 2 | 1.2 | 0.36 | THF | 55 |
| 3 | 1.2 | 0.48 | THF | 62 |
| 4 | 1.2 | 0.60 | THF | 52 |
| 5 | 0.2 | 0.48 | THF | 20 |
| 6 | 0.6 | 0.48 | THF | 52 |
| 7 | 0.8 | 0.48 | THF | 61 |
| 8 | 1.0 | 0.48 | THF | 63 |
| 9 | 1.5 | 0.48 | THF | 52 |
| 10 | 1.2 | 0.48 | Et2O | 53 |
| 11 | 1.2 | 0.48 | 2-Methyl THF | 55 |
| 12 | 1.2 | 0.48 | MTBE | —c |
| 13 | 1.2 | 0.48 | Isopropyl ether | —c |
| 14 | 1.2 | 0.48 | Butyl ether | 83 |
| 15 | 1.2 | 0.48 | Butyl ether | 80d |
| 16 | 1.2 | 0.48 | Butyl ether | 84e |
| 17 | 1.2 | 0.48 | Butyl ether | 87f |
| a Conditions: 1 (1.0 mmol), BF3·OEt2 (1.2 mmol), H2O (0.48 mmol), oil bath of 160 °C, butyl ether (1.0 mL) under argon for 2 h. Isolated yields.b THF as solvent. Oil bath of 120 °C. |
|---|
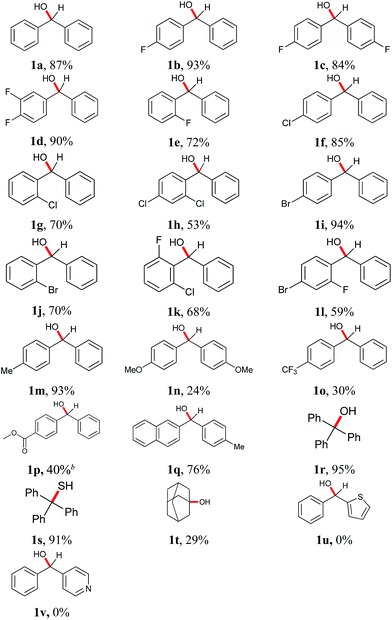 |
With the optimal reaction conditions in hand, diverse substituted diphenylmethanols bearing mono-/di-halo, methyl, and methoxyl groups were investigated with moderate to excellent yields Table 2. Considering the potential utilization of fluorine-containing compounds in pharmaceuticals and functionalized materials,19 fluoro-substituted diphenylmethanols were chosen as substrates such as 4-fluoro- (1b), 4,4′-difluoro- (1c), 3,4-difluoro- (1d) and 2-fluoro- (1e) substituents. As a result, the corresponding alkanes were obtained in yields of 72–93%. The ortho-fluoro substituent (1e) gave a relatively lower yield of 72% than the para-fluoro substitution, probably due to a large extend to the significant steric effects. The chloro- and bromo- substituted aromatics are capable of being further functionalized by SNAr reactions, Grignard reactions and coupling reactions, etc. Structurally diverse chloro- and bromo- substituents (1f–j) were chosen as substrates, and the yields of 53–94% were obtained. It is worthwhile noting that ortho-substitution gave lower yields in the reduction process (1f vs. 1g, h and 1i vs. 1j). When compounds with different halogen groups such as 1k (F, Cl) and 1l (F, Br) were used as substrates, good yields of 68% and 59% were obtained. Diphenylmethanols bearing electron-donating methyl groups such as methyl (1m) and methoxyl (1n) gave the yields of 93% and 24%, respectively. However compared with methyl substituent 1m, dramatically decreased yields were observed by using diphenylmethanol bearing electron-withdrawing trifluoromethyl (1o) and ester (1p) groups. Naphthalene derivative (1q) worked well providing the corresponding product in 76% yield. Interestingly, triphenylmethanol (1r) and triphenylmethanthiol (1s) gave rise to triphenylmethane in 95% and 91%, which suggests that thiol possesses the similar reactivity in this case. Despites many attempts, aliphatic alcohols and aromatic primary or secondary alcohols, did not undergo any reduction process and only adamantanol (1t) generated the corresponding adamentane in an isolated yield of 29%. Heterocyclic substrates (1u and 1v) were disable to give the dehydroxylation products. It is notable that prop-1-ene-1,1-diyldibenzene (2w) was obtained with 99% yield through β-H elimination when 1,1-diphenylpropan-1-ol (1w) was used as substrate, and 1,1-diphenylethanol (1x) only gave ethene-1,1-diyldibenzene (2x) in 55% yield (Scheme 2).
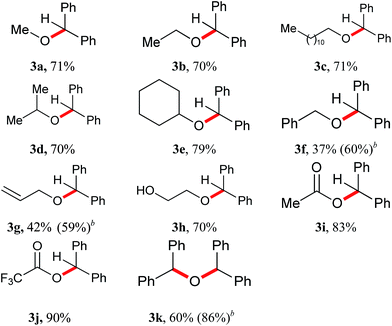
A series of leaving groups has been investigated in reductive reactions as well, such as alkoxy, hydroxymethoxy, and acetyoxy groups (Table 3). The diphenylmethane was produced in yields ranging from 37 to 90% depending on the nature of the leaving group. Methoxy diphenylmethane 3a provided the diphenylmethane in 71% yield. Regardless of linear (3b, 3c), branched (3d) or cyclic (3e) alkoxy diphenylmethyl ethers employed, the reductive products were obtained in good yields. Benzyloxy (3f) and allyloxy (3g) substituted substrates gave similar levels of yields, 37% and 42% respectively, which was probably explained by the released benzylalcohol and allylalcohol which further trap the promoter BF3·OEt2 to form carbonium ion. Interestingly, when doubling the amount of BF3·OEt2 in these two cases, both yields were improved to 60% and 59%. The reaction of the hydroxyethyl substituted substrate (3h) led to 2a in 70% yield, close to the yields obtained for alkoxy substitutions. Both acetoxy (3i) and trifluoroacetoxy (3j) were better leaving groups which resulted in yields of 83% and 90%. Diphenyl ether 3k provided the diphenylmethane in 60% yield under standard conditions. Doubling the amount of BF3·OEt2 improved the yield to 86%.
A large-scale experiment was carried out to demonstrate both the practicality and effectiveness of our method. 74% yield of diphenylmethane (2a) was obtained when treating 2 g of 1a under standard reaction conditions (Scheme 3).
Further experiments were performed to gain a better understanding of the reaction mechanism. Deuterated experiments employing THF-d8 were performed and the results showed the incorporation of the deuterium on the product 2m′ and 92% of D on 2m′, which was readily identified by 1H NMR analysis after silica column chromatography (Scheme 4). It is well-known that the p-σ hyperconjugation exists between the σ electron of α-H of ether and the lone pair electron on oxygen and hence the α-H could be activated through this p-σ hyperconjugation probably resulting in the formation of reductive hydrogen (H−), accompanied with the formation of oxonium ion, which could react with another ether molecule to produce the resonance stabilized species.18c–h Meanwhile, the diphenylmethyl cation could be produced in the presence of BF3 from diphenylmethanol (1). The carbonium ion interacted with reductive hydrogen would give rise to alkane product (2) (Fig. 1).
In summary, we have developed a novel ether/BF3 reductive system for diphenylmethanols and their ether and ester derivatives, presenting a metal-free strategy and affording the corresponding alkane products in good to excellent yields for most cases. The D-labelled experiment showed the reductive hydrogen was generated from ethers. The favourable safety profile, ease to handling and environmentally benign nature make this methodology particularly attractive and practical.
Acknowledgements
We are grateful for funding from the National Natural Science Foundation of China (No. 21372265 and No. 61271059) and sincerely thank Dr Vincent Coeffard for helpful discussions.Notes and references
- (a) A. Yamamoto, Advances in Organometallic Chemistry, ed. F. G. A. Stone and R. West, 1992, vol. 34, p. 111 Search PubMed; (b) R. O. Hutchins, Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Oxford, 1991, vol. 8, p. 327 Search PubMed; (c) S. W. McCombie, Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Oxford, 1991, vol. 8, p. 811 Search PubMed.
- (a) N. Kishner, J. Russ. Phys. Chem. Soc., 1911, 43, 582 Search PubMed; (b) L. Wolff, Justus Liebigs Ann. Chem., 1912, 394, 86 CrossRef PubMed; (c) M. L. Huang, J. Am. Chem. Soc., 1946, 68, 2487 CrossRef CAS.
- (a) G. E. Veitch, E. Beckmann, B. J. Burke, A. Boyer, S. L. Maslen and S. V. Ley, Angew. Chem., Int. Ed., 2007, 46, 7629 CrossRef CAS PubMed; (b) W. C. Liu and C. C. Liao, Chem. Commun., 1999, 117 RSC; (c) S. A. Frank and W. R. Roush, J. Org. Chem., 2002, 67, 4316 CrossRef CAS PubMed.
- (a) D. H. R. Barton and S. W. McCombie, J. Chem. Soc., Perkin Trans. 1, 1975, 1574 RSC; (b) D. H. R. Barton, D. Crich, A. Löbberding and S. Z. Zard, Tetrahedron, 1986, 42, 2329 CrossRef CAS.
- (a) R. M. Lopez, D. S. Hays and G. C. Fu, J. Am. Chem. Soc., 1997, 119, 6949 CrossRef CAS; (b) P. Boussaguet, B. Delmond, G. Dumartin and M. Pereyre, Tetrahedron Lett., 2000, 41, 3377 CrossRef CAS.
- D. A. Spiegel, K. B. Wiberg, L. N. Schacherer, M. R. Medeiros and J. L. Wood, J. Am. Chem. Soc., 2005, 127, 12513 CrossRef CAS PubMed.
- J. M. Herrmann and B. König, Eur. J. Org. Chem., 2013, 7017 CrossRef CAS PubMed.
- Two step procedure by Pd-based catalysts: (a) K. Clauss and H. Jensen, Angew. Chem., Int. Ed., 1973, 12, 918 CrossRef PubMed; (b) G. A. Peterson, F. A. Kunng, J. S. McCallum and W. D. Wulff, Tetrahedron Lett., 1987, 28, 1381 CrossRef CAS; (c) Q. Y. Chen, Y. B. He and Z. Y. Yang, J. Chem. Soc., Chem. Commun., 1986, 1452 RSC; (d) P. Sebök, T. Tímár and T. Eszenyi, J. Org. Chem., 1994, 59, 6318 CrossRef; (e) W. J. Musliner and J. W. Gates Jr., J. Am. Chem. Soc., 1966, 88, 4271 CrossRef CAS; (f) H. Sajiki, A. Mori, T. Mizusaki, T. Ikawa, T. Maegawa and K. Hirota, Org. Lett., 2006, 8, 987 CrossRef CAS PubMed; (g) A. Mori, T. Mizusaki, T. Ikawa, T. Maegawa, Y. Monguchi and H. Sajiki, Tetrahedron, 2007, 63, 1270 CrossRef CAS PubMed; (h) J. M. Saá, M. Dopico, G. Martorell and A. García-Raso, J. Org. Chem., 1990, 55, 991 CrossRef; (i) Y. J. Pan and C. P. Holmes, Org. Lett., 2001, 3, 2769 CrossRef CAS PubMed; (j) A. N. Cammidge and Z. Ngaini, Chem. Commun., 2004, 1914 RSC; (k) J. D. Revell and A. Ganesan, Chem. Commun., 2004, 1916 RSC; (l) H. Kotsuki, P. K. Datta, H. Hayakawa and H. Suenaga, Synthesis, 1995, 1348 CrossRef CAS; (m) H. J. Kim, L. Su, H. Jung and S. Koo, Org. Lett., 2011, 13, 2682 CrossRef CAS PubMed.
- Two step procedure by Ni- and Rh-based catalysts: (a) M. Tobisu, K. Yamakawa, T. Shimasaki and N. Chatani, Chem. Commun., 2011, 47, 2946 RSC; (b) B. H. Lipshutz, B. A. Frieman, T. Butler and V. Kogan, Angew. Chem., Int. Ed., 2006, 45, 800 CrossRef CAS PubMed; (c) P. Álvarez-Bercedo and R. Martin, J. Am. Chem. Soc., 2010, 132, 17352 CrossRef PubMed; (d) H. J. Liu and B. Y. Zhu, Synth. Commun., 1990, 20, 557 CrossRef CAS PubMed.
- (a) S. Sawadjoon, A. Lundstedt and J. S. M. Samec, ACS Catal., 2013, 3, 635 CrossRef CAS; (b) X. H. Liu, G. Z. Lu, Y. L. Guo, Y. Guo, Y. S. Wang and X. H. Wang, J. Mol. Catal. A: Chem., 2006, 252, 176 CrossRef CAS PubMed; (c) M. Dobmeier, J. M. Herrmann, D. Lenoir and B. König, Beilstein J. Org. Chem., 2012, 8, 330 CrossRef CAS PubMed; (d) A. Aramini, M. R. Sablone, G. Bianchini, A. Amore, M. Fanì, P. Perrone, A. Dolce and M. Allegretti, Tetrahedron, 2009, 65, 2015 CrossRef CAS PubMed; (e) P. E. Gordon and A. J. Fry, Tetrahedron Lett., 2001, 42, 831 CrossRef CAS; (f) T. Ankner and G. Hilmersson, Tetrahedron, 2009, 65, 10856 CrossRef CAS PubMed; (g) C. K. Lau, C. Dufresne, P. C. Bélanger, S. Piétré and J. Scheigetz, J. Org. Chem., 1986, 51, 3038 CrossRef CAS; (h) M. A. Tandiary, Y. Masui and M. Onaka, Tetrahedron Lett., 2014, 55, 4160 CrossRef CAS PubMed; (i) H. R. Diéguez, A. López, V. Domingo, J. F. Arteaga, J. A. Dobado, M. M. Herrador, J. F. Quílez del Moral and A. F. Barrero, J. Am. Chem. Soc., 2010, 132, 254 CrossRef PubMed; (j) G. W. Gribble, R. M. Leese and B. E. Evans, Synthesis, 1977, 172 CrossRef CAS; (k) C. F. Nutaitis and J. E. Bernardo, Synth. Commun., 1990, 20, 487 CrossRef CAS PubMed; (l) J. L. Huang, X. J. Dai and C. J. Li, Eur. J. Org. Chem., 2013, 6496 CrossRef CAS PubMed; (m) K. A. Hope-Ross and J. F. Kadla, Can. J. Chem., 2010, 88, 1003 CrossRef CAS; (n) J. L. Fry and M. G. Adlington, J. Am. Chem. Soc., 1978, 100, 7641 CrossRef CAS.
- (a) R. F. Nystrom and C. R. A. Berger, J. Am. Chem. Soc., 1958, 80, 2896 CrossRef CAS; (b) J. E. McMurry, M. G. Silvestri, M. P. Fleming, T. Hoz and M. W. Grayston, J. Org. Chem., 1978, 43, 3249 CrossRef CAS; (c) A. Ono, N. Suzuki and J. Kamimura, Synthesis, 1987, 736 CrossRef CAS.
- (a) M. Yasuda, Y. Onishi, M. Ueba, T. Miyai and A. Baba, J. Org. Chem., 2001, 66, 7741 CrossRef CAS PubMed; (b) M. Mirza-Aghayan, R. Boukherroub and M. Rahimifard, Tetrahedron Lett., 2009, 50, 5930 CrossRef CAS PubMed; (c) H. Wang, L. Li, X. F. Bai, W. H. Deng, Z. J. Zheng, K. F. Yang and L. W. Xu, Green Chem., 2013, 15, 2349 RSC.
- V. Gevorgyan, M. Rubin, S. Benson, J. X. Liu and Y. Yamamoto, J. Org. Chem., 2000, 65, 6179 CrossRef CAS PubMed.
- A. W. Laubengayer and G. R. Finlay, J. Am. Chem. Soc., 1943, 65, 884 CrossRef CAS.
- (a) G. Schäfer and J. W. Bode, Angew. Chem., Int. Ed., 2011, 50, 10913 CrossRef PubMed; (b) G. K. S. Prakash, C. Panja, A. Shakhmin, E. Shah, T. Mathew and G. A. Olah, J. Org. Chem., 2009, 74, 8659 CrossRef CAS PubMed; (c) G. K. S. Prakash, F. Paknia, T. Mathew, G. Mloston, J. P. Joschek and G. A. Olah, Org. Lett., 2011, 13, 4128 CrossRef CAS PubMed; (d) X. F. Xu, Y. Xiong, X. G. Ling, X. M. Xie, J. Yuan, S. T. Zhang and Z. R. Song, Chin. Chem. Lett., 2014, 25, 406 CrossRef CAS PubMed; (e) E. O. Onyango, L. F. Fu and G. W. Gribble, Org. Lett., 2014, 16, 322 CrossRef CAS PubMed; (f) L. X. Shao, Y. P. Zhang, M. H. Qi and M. Shi, Org. Lett., 2007, 9, 117 CrossRef CAS PubMed; (g) L. Shi, Y. Q. Tu, M. Wang, F. M. Zhang, C. A. Fan, Y. M. Zhao and W. J. Xia, J. Am. Chem. Soc., 2005, 127, 10836 CrossRef CAS PubMed; (h) H. Sheng, J. Q. Li, Q. Liu, J. Pan, R. F. Huang and Y. Xiong, J. Org. Chem., 2015, 80, 7212 CrossRef PubMed; (i) J. Q. Li, X. H. Zhang, H. Shen, Q. Liu, J. Pan, W. Hu, Y. Xiong and C. G. Chen, Adv. Synth. Catal., 2015 DOI:10.1002/adsc.201500663 , in press.
- (a) J. A. Cella, J. Org. Chem., 1982, 47, 2125 CrossRef CAS; (b) M. G. Adlington, M. Orfanopoulos and J. L. Fry, Tetrahedron Lett., 1976, 34, 2955 CrossRef; (c) J. L. Fry, M. Orfanopoulos, M. G. Adlington, W. R. Dittman and J. S. B. Silverman, J. Org. Chem., 1978, 43, 374 CrossRef CAS; (d) J. L. Fry and T. J. Mraz, Tetrahedron Lett., 1979, 10, 849 CrossRef.
- (a) Y. Li, Y. Xiong, X. M. Li, X. G. Ling, R. F. Huang, X. H. Zhang and J. C. Yang, Green Chem., 2014, 16, 2976 RSC; (b) S. T. Zhang, X. H. Zhang, X. G. Ling, C. He, R. F. Huang, J. Pan, J. Q. Li and Y. Xiong, RSC Adv., 2014, 4, 30768 RSC; (c) R. F. Huang, X. H. Zhang, J. Pan, J. Q. Li, H. Shen, X. G. Ling and Y. Xiong, Tetrahedron, 2015, 71, 1540 CrossRef CAS PubMed; (d) J. Pan, J. Q. Li, R. F. Huang, X. H. Zhang, H. Shen, X. G. Ling, Y. Xiong and X. M. Zhu, Synthesis, 2015, 47, 1101 CrossRef CAS.
- (a) D. W. A. Sharp and N. Sheppard, J. Chem. Soc., 1957, 674 RSC; (b) K. A. Hofman and H. Kirmreuther, Eur. J. Inorg. Chem., 1909, 42, 4856 Search PubMed; (c) C. E. H. Bawn, R. M. Bell and A. Ledwith, Polymer, 1965, 6, 95 CrossRef CAS; (d) C. E. H. Bawn, R. M. Bell, C. Fitzsimmons and A. Ledwith, Polymer, 1965, 6, 661 CrossRef CAS; (e) I. Kuntz, J. Polym. Sci., Polym. Phys. Ed., 1966, 4, 427 CAS; (f) M. P. Dreyfuss, J. C. Westfahl and P. Dreyfuss, Macromolecules, 1968, 1, 437 CrossRef CAS; (g) K. Din and P. H. Plesch, J. Chem. Soc., Perkin Trans. 2, 1978, 937 Search PubMed; (h) Y. Yamashita, M. Hirota, H. Matsui, A. Hirao and K. Nobutoki, Polym. J., 1971, 2, 43 CrossRef CAS; (i) M. Horn, L. H. Schappele, G. Lang-Wittkowski, H. Mayr and A. R. Ofial, Chem.–Eur. J., 2013, 19, 249 CrossRef CAS PubMed.
- (a) P. Kirsch, Modern fluoroorganic Chemistry, Wiley, Weinheim, 2004 Search PubMed; (b) K. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006 Search PubMed; (c) I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14775a |
| This journal is © The Royal Society of Chemistry 2015 |