Solvatochromism of a tricycloquinazoline based disk-shaped liquid crystal: a potential molecular probe for fluorescence imaging
C. Karthika,
V. Manjuladevi*a,
R. K. Gupta *a and
Sandeep Kumarb
*a and
Sandeep Kumarb
aDepartment of Physics, Birla Institute of Technology and Science, Pilani, Rajasthan – 333031, India. E-mail: manjula@pilani.bits-pilani.ac.in; raj@pilani.bits-pilani.ac.in; Fax: +91 1596 244183; Tel: +91 9828 041535
bRaman Research Institute, Sadashivanagar, Bangalore – 560080, India
First published on 22nd September 2015
Abstract
Tricycloquinazoline (TCQ) based discotic liquid crystals (DLCs) were found to be fluorescent in nature. Such molecules can be employed for the fluorescence imaging of another DLC. Therefore, it is essential to study systematically the photophysical properties of such molecules. In this article, we report the effect of solvents on the photophysical properties of DLC molecules possessing a central TCQ core covalently linked to 6 ethelenoxy side chains (TCQDL molecules). The solutions of TCQDL are prepared in organic solvents of different polarity. The solutions are scanned using ultraviolet-visible (UV-Vis) and fluorescence spectrophotometers. The excited state dipole moment of the TCQDL molecule is estimated from the data. The ground state dipole moment of the TCQDL molecule is calculated using Gaussian 03. We observed that the TCQDL molecule can be used as a fluorophore and used to image other non-fluorescent DLC systems. The epifluorescence imaging of the Langmuir monolayer of the pure TCQDL molecules at the air–water interface showed good image contrast allowing the identification of surface phases even in the absence of additive fluorescent probe molecules.
I. Introduction
The optical imaging of liquid crystals (LCs) provides vital physical insight related to molecular organization and phase transitions.1,2 Various microscopy techniques like near-field scanning optical, fluorescence, fluorescence confocal polarizing, two photon and third harmonic generation microscopy have been employed for such studies.3–12 In all these microscopy techniques, the LC systems have been doped with fluorescent probe molecules. The incorporation of additives may lead to either phase separation or complex formation with the host molecules and thereby may alter the physical properties of the mixed system. So, it is preferable to employ some fluorescent probe with a liquid crystalline nature which can be doped into a similar LC host. Several LC molecules with an intrinsic fluorescence nature have been synthesized and are reported in the literature.13–18Solvatochromism is one of the tools for quantifying the fluorescence response of dye molecules.19 Several dye doped LC systems have been studied through solvatochromism.20–22 In all such reported systems, LCs are doped with dye molecules. The doping of dye molecules may give rise to impurities in the LC system therefore, it is worth studying the solvatochromic behavior of pure LC molecules exhibiting intrinsic fluorescence behavior. Such studies will provide valuable information for LC imaging and display applications. To the our best of knowledge, there are no such studies on pure LC molecules. Discotic liquid crystals (DLCs) possessing a tricycloquinazoline (TCQ) core have been reported to be fluorescent in nature.23,24 We believe that such DLC molecules can potentially be used as molecular probes for fluorescence imaging. Therefore, systematic photophysical studies of such molecules are essential. In this paper, for the first time we report the solvatochromic studies on a DLC molecule possessing a TCQ core with 6 ethelenoxy side chains (TCQDL molecules) without the aid of any dye. Later, we employed TCQDL molecules as fluorescent probes for the optical imaging of another structurally similar DLC system composed of hexa-alkoxy triphenylene (HAT5) molecules. The fluorescence microscopy images reveal the potential of the TCQDL molecules as a probe to study the optical properties of another DLC system. The epifluorescence microscopy technique can be used to visualize the Langmuir monolayer at the air–water interface.25 So, we further extended our studies by performing the epifluorescence imaging of the Langmuir monolayer of pure TCQDL molecules at the air–water interface. We found reasonable contrast in the epifluorescence images which is necessary for identifying phases in the Langmuir monolayer of the TCQDL molecules.
We recorded ultraviolet-visible (UV-Vis) and fluorescence spectra for the TCQDL molecules in various solvents. The solvatochromic shifts were estimated. The ground state and excited state dipole moment of the TCQDL molecule were determined using Lippert’s,26 Bakshiev’s27 and Kawski–Chamma–Viallet’s28,29 equations. Using the density functional theory (DFT) approach, the ground state dipole moment of the TCQDL molecule was computed employing the Gaussian 03 programme package. Calculations were performed to find the parameters like the angular difference between the ground and excited state dipole moments, oscillator strength, transition dipole moment and relative quantum yields of the TCQDL molecules in various solvents.
II. Materials and methods
A. Materials
TCQDL and hexa-alkoxy triphenylene (HAT5) molecules (Fig. 1) were synthesized as reported earlier.30–34 TCQDL exhibits a hexagonal columnar phase (Colh) between 77 and 233 °C.30–32 Upon cooling from the isotropic phase, it displays the classical texture of a columnar mesophase as presented in ref. 30. This compound has been well studied for its conducting and electrochemical properties.30,31 HAT5 was prepared following our previously reported method.33,34 Its phase behavior was in perfect agreement with the reported data.33,34Spectroscopic grade chloroform, ethanol, acetone, dimethylformamide, acetonitrile, dimethylsulfide, methanol, toluene, benzene, butanol, carbontetrachloride, cyclohexane, dichloromethane, diethylether, and tetrahydrofuran were used as procured from Sigma Aldrich and Merck. An optical quality quartz plate was used for the fluorescence imaging of the LC system of HAT5 molecules doped with TCQDL.
B. Experimental methods
The solutions of TCQDL were prepared in various solvents at a fixed solute concentration of 0.25 mM. The UV-Vis spectra were collected using a spectrometer from Jasco (V570). The luminescence spectra were recorded using a spectrofluorimeter (Shimadzu, RF-5301PC) at an excitation wavelength of 390 nm. For the fluorescence imaging of the HAT5 system, chloroform solutions of the mixtures of HAT5 doped with 1, 5 and 10 mole percent of TCQDL were spread onto the quartz plates. The solvent was allowed to evaporate for 60 minutes. The samples prepared were imaged using a fluorescence microscope (BX43 Olympus) in the transmission mode at a temperature of ∼20 °C. The Langmuir monolayer of the TCQDL molecules was formed at the air–water interface35 and was observed under an epifluorescence microscope (Leitz Metallux 3). The images were captured using an intensified charge coupled device camera (Cairn Research). All the experiments were carried out at room temperature (∼20 °C).C. Computational methods
The first principles electronic structure investigation was done on the TCQDL molecule using the Gaussian 03 program package. The geometry optimization of the TCQDL molecule (Fig. 2) was carried out within the DFT framework. This was followed by the evaluation of the vibrational frequency analysis at the B3LYP level of theory. The ground state dipole moment of TCQDL was calculated using the exchange–correlation functional as hybrid B3LYP with the full electron 6-311G basis set.36–38D. Theoretical methods
The ground state and the excited state dipole moment values of the TCQDL molecule were estimated using the following equations.Lippert’s equation:
| νa − νf = mF(k, n) + C | (1) |
Bakshiev’s equation:
| νa − νf = m1F1(k, n) + C | (2) |
Kawski–Chamma–Viallet’s equation:
| (νa + νf)/2 = −m2F2(k, n) + C | (3) |
Lippert’s polarity function:
 | (4) |
Bakshiev’s polarity function:
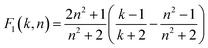 | (5) |
Kawski–Chamma–Viallet’s polarity function:
 | (6) |
Slopes m and m1 are estimated from the plots of F(k, n), F1(k, n) versus Stokes shift respectively. The slope m2 is estimated from the plot of F2(k, n) vs. the arithmetic mean of the absorption and fluorescence wavenumber.26–29 The values of the slopes m, m1 and m2 can be further related to the ground state dipole moment (pg), excited state dipole moment (pe), Onsager radius cavity (a), Planck constant (h) and speed of light in a vacuum (c) by following equations:
 | (7) |
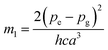 | (8) |
 | (9) |
These equations are based on the Onsager reaction field theory, which assumes that the fluorophore is a point dipole residing in the center of a spherical cavity in a homogeneous and isotropic dielectric medium with a relative permittivity k. The cavity radius chosen for our study is the radius of the TCQ core (=0.5 nm) of the TCQDL molecule.23,39
Furthermore, the ground state and excited state dipole moments can also be calculated using the following equations:
 | (10) |
 | (11) |
From eqn (10) and (11) we get:
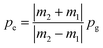 | (12) |
Generally the excited state dipole moment and the ground state dipole moment are not parallel to each other. So, the angular separation (ϕ) between them is estimated by the following relation40,41
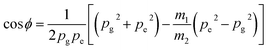 | (13) |
Further, from the fluorescence spectra, the relative quantum yield, oscillator strength and transition dipole moment of the TCQDL molecule are estimated. In the fluorescence spectra, the maximum intensity was observed for the case of the TCQDL molecule in dichloromethane. So, the relative quantum yield 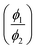 can be calculated from the quantum yield of the TCQDL molecule in various solvents (ϕ1) with respect to that of the TCQDL molecule in dichloromethane (ϕ2) using the following equation:42
can be calculated from the quantum yield of the TCQDL molecule in various solvents (ϕ1) with respect to that of the TCQDL molecule in dichloromethane (ϕ2) using the following equation:42
 | (14) |
The oscillator strength (f) of the TCQDL molecule in various solvents is computed using the following equation:43
| f = 4.32 × 10−9εmaxν1/2 | (15) |
The transition dipole moment of the TCQDL molecule is obtained using the approximated equation below:44
 | (16) |
III. Results and discussion
The absorption and the corresponding fluorescence spectra for the TCQDL molecules in different solvents are shown in Fig. 3. The values for absorption and the fluorescence maxima wavenumbers were calculated from the spectra. The UV-Vis spectroscopic measurements show an average absorption maximum at 402 nm. The luminescence spectra were recorded at an excitation wavelength of 390 nm. | ||
| Fig. 3 The UV-Vis (left side) and fluorescence (right side) spectra of the TCQDL molecules in various solvents. | ||
Fig. 3 shows that the solvent-induced shift of the emission spectrum is larger than the shift in the absorption spectrum. The shift observed in the spectral band arises from the large reaction field which a solute molecule experiences due to the polarization of the surrounding solvent molecules.45 This is due to the larger excited-state permanent dipole moment of the TCQDL molecule when compared to its ground state. The shifts observed are dependent on the nature of solvent (polar or non-polar) and dielectric constant. Since in the present case, the excited state of a molecule is very different from its ground state, the shift may be more or less depending upon the transition involved. The ground state dipole moment may slightly change from solvent to solvent, but the excited state may be very different. Thus it is expected that there may be small changes in the absorption, while large changes in the fluorescence spectrum are seen with a change in the solvent.
Table 1 shows the absorption wavenumbers (νa), emission wavenumbers (νf), Stokes shift (νf − νa) and arithmetic means of the emission and absorption wavenumbers ((νf + νa)/2) of the TCQDL molecule in different solvents. The Stokes shift is an indicator of the change from the ground state to the excited state structure of the TCQDL molecule. This can be attributed to factors such as the dipole–dipole interaction between the solvent and solute, the change in the nature of the emitting state induced by the solvent and the specific solvent–solute interactions such as hydrogen bonding.46 The Stokes shift also defines the change in the energies of the levels of a solute due to the change in the solvent medium. Emission states arise out of the energy of more relaxed excited states and hence are more informative than absorption spectra. The parameters found using these shifts are vital for describing the intramolecular charge transfer in the molecular excited states47–51 and the inter-molecular charge transfer in the excited complexes.52,53
| Solvents used | νf (cm−1) | νa (cm−1) | νf − νa (cm−1) | (νf + νa)/2 (cm−1) |
|---|---|---|---|---|
| Chloroform | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073.37 073.37 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.01 517.01 |
1443.64 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 795.19 795.19 |
| Acetone | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 397.29 397.29 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738.39 738.39 |
1341.11 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067.84 067.84 |
| Acetonitrile | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408.24 408.24 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738.39 738.39 |
1330.15 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073.32 073.32 |
| Dimethylformamide | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105.36 105.36 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738.39 738.39 |
1633.03 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 921.88 921.88 |
| Toluene | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126.73 126.73 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.01 517.01 |
1390.28 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821.87 821.87 |
| Dichloromethane | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126.73 126.73 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.01 517.01 |
1390.28 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821.87 821.87 |
| Carbontetrachloride | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141.18 141.18 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.01 517.01 |
1375.83 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 829.1 829.1 |
| Tetrahydrofuran | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786.31 786.31 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738.39 738.39 |
1952.08 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762.35 762.35 |
In the present study, non-polar (k < 5), borderline polar aprotic (k ranges from 5–20) and polar aprotic (k > 20) solvents were employed. We observed bathochromic shifts (red shifts) from the ground to the excited state of TCQDL in solvents of different polarity. Due to the efficiency of the vibrational relaxation, there is a loss of energy before the fluorescence emission. This loss of energy results from several dynamic processes, including the dissipation of vibrational energy, reorientation of the solvent molecules around the excited state dipole, redistribution of the electrons in the solvent molecules as a result of the altered dipole moment of the excited fluorophore, and fluorophore–solvent interactions (such as hydrogen bonding). The shifts observed for the TCQDL molecule in various solvents indicate that the transition involved is from a π-bonding orbital to an antibonding (π*) orbital (π → π* transition) and the lowest lying state of the TCQDL molecule is π → π*.
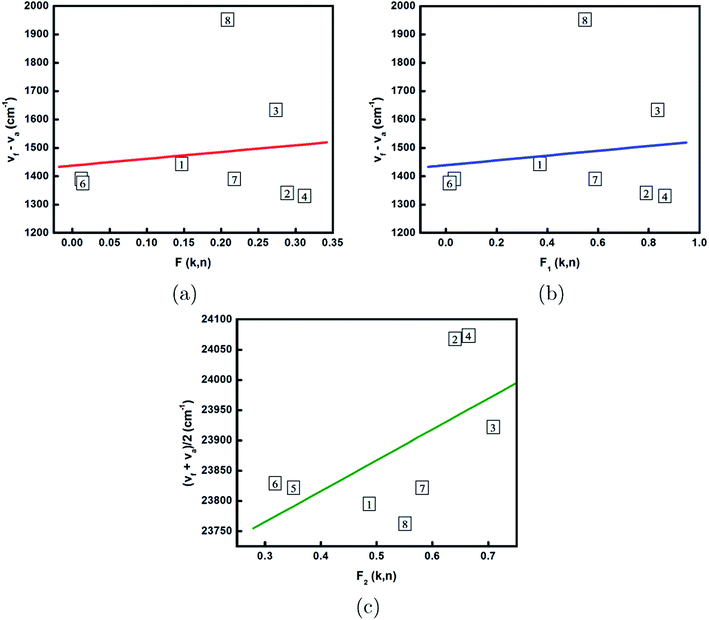
Using eqn (4)–(6), Lippert’s, Bakshiev’s and Kawski–Chamma–Viallet’s polarity functions for various solvents (Table 2) are calculated. The k and n values were taken from the literature.29 In Fig. 4, the plot (a) shows the Stokes shift versus F(k, n); (b) shows the Stokes shift versus F1(k, n); and (c) shows the arithmetic mean of the emission and absorption wavenumbers versus F2(k, n) for the TCQDL molecule in various solvents. The linear fits of the spectral shifts on the polarity functions (Fig. 4) have positive slopes.
| Solvents used | k | n | F(k, n) | F1(k, n) | F2(k, n) |
|---|---|---|---|---|---|
| Chloroform | 4.81 | 1.442 | 0.147 | 0.371 | 0.487 |
| Acetone | 21.09 | 1.359 | 0.289 | 0.791 | 0.641 |
| Acetonitrile | 36.64 | 1.344 | 0.312 | 0.863 | 0.665 |
| Dimethylformamide | 36.7 | 1.431 | 0.274 | 0.835 | 0.709 |
| Toluene | 2.38 | 1.497 | 0.012 | 0.033 | 0.351 |
| Dichloromethane | 8.93 | 1.424 | 0.218 | 0.589 | 0.582 |
| Carbon tetrachloride | 2.24 | 1.459 | 0.014 | 0.015 | 0.318 |
| Tetrahydrofuran | 7.58 | 1.407 | 0.209 | 0.549 | 0.551 |
From Fig. 4, the slopes m, m1 and m2 were estimated to be 239.2, 84.8 and 509.3 cm−1, respectively. Using eqn (10), the ground state dipole moment (pg) value of the TCQDL molecule was found to be 2.56 D. The excited dipole moment (pe) of the TCQDL molecule was found to be 3.59 D (Table 3). The higher value of the excited state dipole moment compared to that of the ground state of TCQDL might be due to an increase in charge separation in the excited state. The change in the excited state dipole moment with reference to the ground state dipole moment (δp) i.e. pe − pg and the ratio of the excited state to the ground state dipole moments  of the TCQDL molecule were found to be 1.03 D and 1.40, respectively. It can be seen that the pe value obtained for the TCQDL molecule using the Lippert method is large compared to the values obtained by all other methods (Table 3). This is due to the fact that this method does not take into account the polarizability of the solute. It is observed that the shift of the emission peak with the change in solvent polarity is more pronounced than the shift of the absorption peak, indicating pe > pg; i.e. the dipole moments of the molecules increase on excitation. Therefore, the excited state of TCQDL is more polar than the ground state.
of the TCQDL molecule were found to be 1.03 D and 1.40, respectively. It can be seen that the pe value obtained for the TCQDL molecule using the Lippert method is large compared to the values obtained by all other methods (Table 3). This is due to the fact that this method does not take into account the polarizability of the solute. It is observed that the shift of the emission peak with the change in solvent polarity is more pronounced than the shift of the absorption peak, indicating pe > pg; i.e. the dipole moments of the molecules increase on excitation. Therefore, the excited state of TCQDL is more polar than the ground state.
| Calculations | pg (D) | pe (D) | pe/pg | ϕ |
|---|---|---|---|---|
| Estimated | 2.56 | 3.59 | 1.40 | 0° |
| Gaussian’03 | 3.20 | |||
| Lippert’s theory | 4.28 | |||
| Bakshiev’s theory | 3.85 | |||
| Kawski–Chamma–Viallet’s theory | 3.85 |
Using Gaussian 03, the ground state dipole moment value of the TCQDL molecule was computed and it was found to be 3.2 D. The difference in the estimated and computed values of the dipole moment is due to the fact that the molecule is considered as an isolated system (as in the gas phase) during the computation, whereas the experimentally obtained values are in the solution phase, where the solvent (matrix) introduces a strong perturbation. Further it is evident from Table 3 that the changes in the dipole moments of the TCQDL molecule on electronic excitation are rather small. This suggests that the emission of the TCQDL molecule originates from a state, which although more polar than the ground state, is probably a locally excited intramolecular charge transfer (ICT) state. Charge transfer accompanying excitation to the lowest excited singlet state usually results in the excited molecule having a greater dipole moment than the ground state.54 Usually during the estimation of these parameters, it is generally assumed that the excited state dipole moment is almost parallel with the ground state.55–58 Accordingly using eqn (13), we found no angular separation between the excited and the ground state dipole moment of the TCQDL molecule (i.e. 0°). Hence, the dipole moments in both the states were found to be collinear.
The polarity of a solvent generally influences the fluorescence-emission spectra of the fluorophores. Changes in the quantum yields and shifts in spectra are valuable parameters of fluorophore sensitivity to the solvent polarity. The quantum yield is the ratio of the total number of emitted photons to the total number of photons absorbed. The sensitivity of the fluorophores to the solvent polarity has practical applications in the field of physical biochemistry. When fluorophores are bound to proteins, nucleic acids, membranes, or macromolecules, in general, the fluorescence-emission spectra change. These changes can be employed to detect binding sites on macromolecules or to determine the polarity of binding sites.59
The estimated values of the relative quantum yield of TCQDL in various solvents are shown in Table 4. The estimated values indicated a maximum quantum yield for TCQDL in toluene with respect to that of TCQDL in dichloromethane and a minimum quantum yield for TCQDL in acetonitrile with respect to that of TCQDL in dichloromethane. The hydrogen-bonded solvents generally show a greater red shift than those which do not form hydrogen bond. Hence, larger quantum yields are observed in solvents with no hydrogen bonding. The estimated relative quantum yield value of TCQDL in acetonitrile is very low which could be due to emissionless deactivation processes during the transition from the excited state to the ground state.
| Solvents used | A | A* (390 nm) | P | ϕ1/ϕ2 |
|---|---|---|---|---|
| Chloroform | 0.27 | 0.22 | 4.1 | 0.2 |
| Acetone | 0.26 | 0.22 | 5.1 | 0.1 |
| Acetonitrile | 0.21 | 0.18 | 5.8 | 0.014 |
| Dimethylformamide | 0.24 | 0.2 | 6.4 | 0.084 |
| Toluene | 0.21 | 0.16 | 2.4 | 0.754 |
| Dichloromethane | 0.25 | 0.21 | 3.1 | 1 |
| Carbontetrachloride | 0.22 | 0.17 | 1.6 | 0.29 |
The oscillator strength (f), a measure of the integrated intensity of the charge transfer complex and the transition dipole moment (μt) of the TCQDL molecules in various solvents, are listed in Table 5. The values of the oscillator strength indicate a strong interaction between the donor–acceptor pair with relatively high probability transitions in the TCQDL molecule. The maximum oscillator strength of the TCQDL molecule was found to be in tetrahydrofuran.
| Solvents used | εmax | Δνa (cm−1) | f | μt (D) |
|---|---|---|---|---|
| Chloroform | 864 | 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508.7 508.7 |
0.539 | 6.84 |
| Acetone | 832 | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671.8 671.8 |
0.548 | 6.86 |
| Dimethylformamide | 672 | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671.8 671.8 |
0.443 | 6.17 |
| Acetonitrile | 768 | 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362.6 362.6 |
0.455 | 6.26 |
| Toluene | 672 | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671.8 671.8 |
0.443 | 6.2 |
| Carbontetrachloride | 800 | 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362.6 362.6 |
0.474 | 6.41 |
| Dichloromethane | 704 | 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508.7 508.7 |
0.439 | 6.17 |
| Tetrahydrofuran | 896 | 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508.7 508.7 |
0.559 | 6.93 |
The transition dipole moments of the TCQDL molecule in various solvents from the absorption data are listed in Table 5. They are based on the assumption of complete transfer of one unit of charge in the transition. It is reasonable to assume that this represents a maximum in the amount of charge that might be separated, and therefore a minimum length for the charge separation.44,60 The estimated values ranged from 6.17–6.93 D. The transition dipole moment values for TCQDL in dimethylformamide and dichloromethane were found to be same (Fig. 5).
In our study, we found the solvatochromic shifts without the aid of any inorganic or organic dye. This is an indicator that the TCQDL molecule can act as a fluorophore, and hence does not require any dye to aid solvatochromism. In order to employ TCQDL molecules for fluorescence imaging, we doped another DLC system of HAT5 molecules with 1, 5 and 10 mole percent of TCQDL molecules. The fluorescence image of the pure TCQDL molecule reveals a bright green uniform region (Fig. 6a). However the fluorescence image of the pure HAT5 shows a completely dark region indicating no signature of fluorescence (inset in (Fig. 6a)). The image corresponding to 1% of TCQDL in HAT5 (Fig. 6b) shows a bright green texture and dark regions. As both the TCQDL and HAT5 exhibit a colh mesophase, they are well miscible in all proportions. The dark regions in Fig. 6c and d are voids. The brightness of the green region increases with the increase in the concentration of TCQDL in HAT5 (Fig. 6c and d). This indicates that even with 1 mole percent of TCQDL in HAT5, the imaging of a LC system is possible.
In order to observe the Langmuir monolayer of the TCQDL molecules at the air–water interface, we have carried out epifluorescence microscopy25 without the aid of any fluorophore. The epifluorescence images were captured at the points A and B as indicated in the isotherm of the TCQDL monolayer (Fig. 7a) and they are shown in Fig. 7b. Both the figures show bright regions indicating the fluorescent feature of the TCQDL molecules. Fig. 7b(A) shows a bright region and dark regions. The bright region is due to the liquid-like phase of the TCQDL molecules whereas the dark regions indicate the gas phase. On compression the dark region disappears leading to a uniform bright region. This indicates that on compression of the monolayer, the gas phase disappears and a uniform liquid-like phase appears (Fig. 7b(B)). Such observation further confirms that the TCQDL molecules can be employed for various fluorescence studies.
IV. Conclusion
The effect of various solvents of different polarity on the photophysical properties of the TCQDL molecule was studied. We observed a red shift in the emission spectra with an increase in the polarity of the solvent. The Stokes shift, and Lippert’s, Bakshiev’s, and Kawski–Chamma–Viallet’s polarity functions were estimated from the experimental data. We found the ground and excited state dipole moment values of the molecule to be 2.56 D and 3.59 D respectively. Computationally, the ground state dipole moment value was calculated using the Gaussian 03 package and it was found to be 3.20 D.Our study indicates that the TCQDL molecule can be employed as a fluorophore for imaging another non-fluorescent DLC. It will be interesting to study the photophysical properties of such molecules in a confined geometry formed by different mesophases of LCs. This shows the potential of TCQ based LCs for imaging using confocal and two-photon microscopy.
Acknowledgements
The authors (BITS, Pilani) are thankful to University Grants Commission of India for their support under UGC SAP programme. CK thanks UGC for BSR fellowship. CK thanks Kuldeep Gupta, research scholar (BITS, Pilani) for helping with fluorescence microscopy. We sincerely thank reviewers for their valuable suggestions.References
- B. L. Feringa, Molecular Switches, Wiley, Weinheim, 2001 Search PubMed.
- M. C. Petty, M. R. Bryce and D. Bloor, An Introduction to Molecular Electronics, Oxford University Press, USA, 1995 Search PubMed.
- D. A. Higgins, X. Liao, J. E. Hall and E. Mei, J. Phys. Chem. B, 2001, 105, 5874–5882 CrossRef CAS.
- K. Amundson, A. van Blaaderen and P. Wiltzius, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1997, 55, 1646–1654 CrossRef CAS.
- M. Ofuji, Y. Takano, Y. Houkawa, Y. Takanishi, K. Ishikawa, H. Takezoe, T. Mori, M. Goh, S. Guo and K. Akagi, Jpn. J. Appl. Phys., 2006, 45, 1710–1713 CrossRef CAS.
- G. A. Held, L. L. Kosbar, I. Dierking, A. C. Lowe, G. Grinstein, V. Lee and R. D. Miller, Phys. Rev. Lett., 1997, 79, 3443–3446 CrossRef CAS.
- O. D. Lavrentovich, Pramana, 2003, 61, 373–384 CrossRef CAS.
- P. S. Salter, G. Carbone, E. J. Botcherby, T. Wilson, S. J. Elston and E. P. Raynes, Phys. Rev. Lett., 2009, 103, 257803 CrossRef CAS.
- W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73–76 CAS.
- R. S. Pillai, M. OhE, H. Yokoyama, G. J. Brakenhoff and M. Muller, Opt. Express, 2006, 14, 12976–12983 CrossRef CAS.
- D. Yelin, Y. Silberberg, Y. Barad and J. S. Patel, Appl. Phys. Lett., 1999, 74, 3107–3109 CrossRef CAS PubMed.
- D. Yelin, Y. Silberberg, Y. Barad and J. S. Patel, Phys. Rev. Lett., 1999, 82, 3046–3049 CrossRef CAS.
- R. D. Larrabee, US Pat., US3960753A, 1976.
- S. Benning, H.-S. Kitzerow, H. Bock and M.-F. Achard, Liq. Cryst., 2000, 27, 901–906 CrossRef CAS PubMed.
- S. Kim and S. Y. Park, Mol. Cryst. Liq. Cryst., 1999, 337, 405–408 CrossRef CAS PubMed.
- P. B. Hoag and D. L. Gin, Adv. Mater., 1998, 10, 1546–1551 CrossRef.
- M. Salamonczyk, A. Kovarova, J. Svoboda, D. Pociecha and E. Gorecka, Appl. Phys. Lett., 2009, 95, 171901 CrossRef PubMed.
- A. C. Sentman and D. L. Gin, Adv. Mater., 2001, 13, 1398–1401 CrossRef CAS.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, John Wiley and Sons, New York, 2011 Search PubMed.
- L. Marrucci, D. Paparo, M. R. Vetrano, M. Colicchio, E. Santamato and G. Viscardi, J. Chem. Phys., 2000, 113, 10361–10366 CrossRef CAS PubMed.
- H. Iwanaga, K. Naito and F. Effenberger, Liq. Cryst., 2000, 27, 115–123 CrossRef CAS PubMed.
- A. G. Gilani, M. Yazdanbakhsh, N. Mahmoodi, M. Moghadam and E. Moradi, J. Mol. Liq., 2008, 139, 72–79 CrossRef PubMed.
- E. Keinan, S. Kumar, S. P. Singh, R. Ghirlando and E. J. Wachtel, Liq. Cryst., 1992, 11, 157–173 CrossRef CAS PubMed.
- S. Kumar, Chemistry of DLCs: From Monomers to Polymers, CRC Press, Boca Raton, 2011 Search PubMed.
- V. von Tscharner and H. M. McConnell, Biophys. J., 1981, 36, 409–419 CrossRef CAS.
- E. Lippert, Z. Naturforsch., 1955, 10a, 541–545 CAS.
- N. G. Bakshiev, O. P. Girin and V. S. Libov, Opt. Spectrosc., 1963, 14, 395–398 Search PubMed.
- A. Kawski, Z. Naturforsch., A: Phys. Sci., 2002, 57, 255–262 CrossRef CAS.
- A. Chamma and P. Viallet, C. R. Acad, Sci. Paris, Ser. C, 1970, 270, 1901–1904 CAS.
- N. Boden, R. J. Bushby, K. Donovan, Q. Liu, Z. Lu, T. Kreoouzis and A. Wood, Liq. Cryst., 2001, 28, 1739–1748 CrossRef CAS PubMed.
- R. J. Bushby, O. R. Lozman, L. A. Mason, N. Taylor and S. Kumar, Mol. Cryst. Liq. Cryst., 2004, 410, 171–181 CrossRef PubMed.
- S. Kumar, D. S. S. Rao and S. K. Prasad, J. Mater. Chem., 1999, 9, 2751–2754 RSC.
- S. K. Pal, S. Setia, B. S. Avinash and S. Kumar, Liq. Cryst., 2013, 40, 1769–1816 CrossRef CAS PubMed.
- S. Kumar and S. K. Varshney, Synthesis, 2001, 305–311 CrossRef CAS.
- C. Karthik, V. Manjuladevi, R. K. Gupta and S. Kumar, J. Mol. Struct., 2014, 1070, 52–57 CrossRef CAS PubMed.
- J. Montgomery Jr, M. Frisch, J. W. Ochterski and G. A. Petersson, J. Chem. Phys., 1999, 110, 2822–2827 CrossRef PubMed.
- P. Cimino, L. Gomez-Paloma, D. Duca, R. Riccio and G. Bifulco, Magn. Reson. Chem., 2004, 42, S26–S33 CrossRef CAS PubMed.
- R. Jain, T. Bally and P. R. Rablen, J. Org. Chem., 2009, 74, 4017–4023 CrossRef CAS PubMed.
- R. K. Gupta, V. Manjuladevi, C. Karthik, S. Kumar and K. Suresh, Colloids Surf., A, 2012, 410, 91–97 CrossRef CAS PubMed.
- P. Suppan, Chem. Phys. Lett., 1983, 94, 272–275 CrossRef CAS.
- Y. H. Zhao, M. H. Abraham and A. M. Zissimos, J. Org. Chem., 2003, 68, 7368–7373 CrossRef CAS PubMed.
- J. V. Morris, M. A. Mahaney and J. R. Huber, J. Phys. Chem., 1976, 80, 969–974 CrossRef CAS.
- H. Tsubomura and R. P. Lang, J. Am. Chem. Soc., 1961, 83, 2085–2092 CrossRef CAS.
- P. Venuvanalingam, U. C. Singh and N. R. Subbaratnam, Spectrochim. Acta, 1981, 37, 505–510 CrossRef.
- J. R. Lombardi, J. Phys. Chem. A, 1998, 102, 2817–2823 CrossRef CAS.
- G. Gisha, Photochemical and Photophysical Studies of a Few Bischromophoric Systems, PhD thesis, Cochin University of Science and Technology, Kerala, India, 2010.
- M. B. Ledger and P. Suppan, Spectrochim. Acta, Part A, 1967, 23, 641–653 CrossRef CAS.
- P. Suppan, J. Chem. Soc. A, 1968, 3125–3133 RSC.
- M. Ito, K. Inuzuka and M. Imanishi, J. Am. Chem. Soc., 1960, 82, 1317–1322 CrossRef CAS.
- D. K. Deshpande, M. A. Shashidhar and K. S. Rao, Z. Phys. Chem., 1981, 262, 588–592 CAS.
- L. S. Prabhumirashi, D. K. N. Kutty and A. S. Bhide, Spectrochim. Acta, Part A, 1983, 39, 663–668 CrossRef.
- Z. R. Grabowski, K. Rotkiewicz, A. Siemiarezuk, D. J. Cowley and W. Baumann, Nouv. J. Chim., 1979, 3, 443–454 CAS.
- H. Beens, H. Knibbe and A. Weller, J. Chem. Phys., 1967, 47, 1183–1184 CrossRef CAS PubMed.
- V. K. Sharma, P. D. Saharo, N. Sharma, R. C. Rastogi, S. K. Ghoshal and D. Mohan, Spectrochim. Acta, Part A, 2003, 59, 1161–1170 CrossRef.
- B. Siddlingeshwar and S. M. Hanagodimath, Spectrochim. Acta, Part A, 2009, 72, 490–495 CrossRef CAS PubMed.
- J. Kabatac, B. Osmialowski and J. Paczkowski, Spectrochim. Acta, Part A, 2006, 63, 524–531 CrossRef PubMed.
- D. S. Biradar, B. Siddlingeshwar and S. M. Hanagodimath, J. Mol. Struct., 2008, 875, 108–112 CrossRef CAS PubMed.
- J. Thipperudrappa, D. S. Biradar, S. R. Manohara, S. M. Hanagodimath, S. R. Inamadar and R. J. Manekutla, Spectrochim. Acta, Part A, 2008, 69, 991–997 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Press, New York, 1986 Search PubMed.
- M. S. Paley, J. M. Harris, H. Looser, J. C. Baumert, G. C. Bjorklund, D. Jundt and R. J. Twieg, J. Org. Chem., 1989, 54, 3774–3778 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |