Aqueous biphasic separation of 97Ru and 95,96Tc from yttrium
Moumita Maitia,
Arpita Datta†
b and
Susanta Lahiri*b
aDepartment of Physics, Indian Institute of Technology Roorkee, Roorkee-247667, Uttarakhand, India
bChemical Sciences Division, Saha Institute of Nuclear Physics, 1/AF Bidhannagar, Kolkata 700064, India. E-mail: susanta.lahiri@saha.ac.in; Tel: +91-9433988997
First published on 9th September 2015
Abstract
An aqueous biphasic separation technique has been developed for the separation of 97Ru, a potential candidate radionuclide in nuclear medicine, from its target matrix, yttrium. The extraction of ruthenium and technetium from bulk yttrium has been carried out with 50% (w/v) PEG-4000 and PEG-6000 against 2 M solution of various salts, such as Na-citrate, Na-tartarate, Na-malonate, Na2CO3, NaHSO3, Na2SO4, Na2S2O3, K2HPO4, K3PO4, (NH4)2SO4 and 4 M KOH, at room temperature. The influence of the pH of some salt rich phases (e.g., Na-tartarate and (NH4)2SO4) on the extraction behavior of 97Ru and 95,96Tc into the PEG rich phase was also studied. In the presence of Na-tartarate, Na-citrate, K2HPO4, K3PO4, KOH, Na2CO3, Na2SO4, Na2SO3 and (NH4)2SO4 salt solutions, 97Ru and 95,96Tc were preferentially extracted into the PEG rich phase. In 50% (w/v) PEG-4000-2 M (NH4)2SO4 ABS system, 83% of 97Ru along with 96% of 95,96Tc were extracted into the PEG rich phase without any contamination of the yttrium target. Back extraction of 97Ru into the salt rich phases from the PEG rich phase was also carried out using 2 M salt solutions of K2CO3, Na2S2O3 and 4 M KOH. About 90% back extraction of 97Ru into the salt rich phases without any contamination of 95,96Tc was obtained with 2 M Na2S2O3 salt solution. Dialysis of the PEG rich phase containing 97Ru along with 95,96Tc was also carried out against deionised water to obtain pure 97Ru.
Introduction
Polyethylene glycol (PEG) based aqueous biphasic systems (ABS) are greener analytical techniques compared to traditional solvent extraction systems, because both the phases in ABS are aqueous in nature.1 ABS can be formulated with the water-soluble polymer PEG and other polymers or PEG with inorganic salts, such as sulphate, phosphate, and carbonate, in particular concentrations.2 The properties of ABS generally depend on the phase components of the ABS system. PEG can be chosen as one of the phase forming components because of its nontoxic, non-flammable, and inexpensive nature.3–5 In addition, the PEG-rich phase in a PEG-ABS system is tunable and, therefore, the phase characteristics of such systems can be modified by fine tuning the PEG-rich phase for better partitioning behavior of the solute into the PEG-rich phase.Various applications of ABS systems in the fields of separation and purification of organic molecules and metal ions have been reported in the literature. Roger et al. reported the partitioning behavior of pertechnetate using a PEG-ABS system.3,5,6 Over the last ten years, our laboratory has made continuous endeavors to develop new green separation methodologies, using aqueous biphasic systems7–16 or other environmentally benign reagents such as polyvinylpyrrolidone17,18 and ionic liquids.19
In this study, we made an attempt to separate Ru and Tc radionuclides from bulk yttrium target. Radiometric methods were employed for detection. Corresponding radioisotopes, such as 97Ru, 95,96Tc and 88Y, were used as the precursors of Ru, Tc and Y, respectively. The motivation of the experiment lies in the fact that 97Ru is a candidate radionuclide in nuclear medicine, which may have potential applications in diagnostic imaging as well as for therapeutic purposes because of its suitable chemical and nuclear properties, such as a moderate half-life (T1/2: 2.83 d) and high intensity low energy γ rays (216 keV, 86% and 324.5 keV, 10.25%). Due to the presence of multiple oxidation states such as Ru(II), Ru(III), Ru(IV) and Ru(VIII) and various coordination numbers (4, 5 and 6), Ru can form a series of complexes that have useful properties for tuning various metal-ligand combinations for radiopharmaceutical chemistry.20 Generally, the reported production routes of 97Ru are neutron, proton and alpha particle activation on suitable targets.21–31 Recently, we reported two new production routes of 97Ru by heavy ion activation such as activation through natNb(7Li, 3n)97Ru32 and natY(12C, p3n)97Ru reactions.33 In the latter reaction, i.e., by bombarding a yttrium target with 75 MeV 12C, 97Ru and 95,96Tc are produced in the target matrix.
The separation of 97Ru from the corresponding targets were reported using various analytical techniques such as solvent extraction, dry distillation, co-precipitation, wet distillation, liquid–liquid extraction and solid–liquid extraction methods. The separation of 97Ru from Tc and Rh targets using a distillation technique based on the distillation of 97RuO4 in concentrated HNO3 or H2SO4 medium at 90 °C has been reported in the literature with a total separation time of 6–7 h.21,22,25 Comar et al. developed and described a solvent extraction process that is simple and rapid compared to distillation processes for the separation of Ru and co-produced Tc radionuclides from a molybdenum target.28 A tin dioxide column followed by an anion exchange column was employed for the separation of 97Ru from bulk molybdenum target.31 Liquid–liquid extraction (LLX) using a liquid anion exchanger, trioctylamine (TOA), or a liquid cation exchanger, di-(2-ethylhexyl)phosphoric acid (HDEHP) along with tri-butyl phosphate (TBP) was used for the separation of 97Ru from coproduced Tc and Nb radionuclides and bulk Mo target by Lahiri et al.34,35 The radiochemical separation of NCA 97Ru from bulk Nb and coproduced Tc by LLX using both HDEHP and SLX with a cation exchanger resin, DOWEX-50, was exploited by Maiti et al.32 Maiti et al. also reported the separation of 97Ru and coproduced 95Tc from bulk yttrium target by LLX using TOA.33 Recently, we developed a PEG-based aqueous biphasic system for the separation of 97Ru from bulk niobium target.12 We also developed a method of separation of 97Ru from 12C induced natural yttrium target by ion exchange resins.36 In this study, we have made an attempt to develop another green method for the separation of 97Ru from 12C-induced bulk Y target and co-produced 95,96Tc using a PEG based ABS system.
Materials and methods
Irradiation
Dictated by theoretical calculations,33 we bombarded natural Y foil (99.9% purity, Alfa Aesar) with 75 MeV of 12C6+ beam for 14 h at the BARC-TIFR Pelletron facility, Mumbai for the production of 97Ru and 95,96Tc.33,37 The bulk Y target was monitored radiometrically using 88Y. Gamma-spectroscopic measurements were carried out using an HPGe (CANNBERA) detector with 2.7 keV resolution at 1332 keV. After the end of bombardment (EOB), the 12C irradiated natY target was cooled for 50 h to allow the decay of all short-lived products. The carbon irradiated natY foil was dissolved in a minimum volume of 0.1 M HCl and was spiked with 88Y, evaporated to dryness, and re-dissolved in 0.01 M HCl to prepare the stock solution containing 97Ru, 95,96Tc and 88Y along with bulk Y.Materials and procedure
Chemicals, such as PEG-4000, PEG-6000, HNO3, HCl, and HF, and salts, such as Na-citrate, Na-tartarate, Na-malonate, (NH4)2SO4, NaHSO3, Na2SO4, Na2SO3, Na2S2O3, K2HPO4, K3PO4, Na2CO3, and KOH, were obtained from Merck, India. All the reagents were of analytical grade. The dialysis sack was procured from Spectrum Laboratory Inc.The molecular weight and concentration of PEG, the salt concentration and the type of salts employed are important parameters to obtain maximum phase separation in ABS systems. PEG-4000 with 50% (w/v) concentration is reported as an optimum condition to minimize the solubility of any salt rich phase in the polymer rich phase.9,38 Moreover, it has been found that 2–4 M salt concentration is ideal to obtain maximum phase separation. In the present study, therefore, 50% (w/v) PEG-4000 solution and 2 M salt solutions of Na-citrate, Na-tartarate, Na-malonate, (NH4)2SO4, NaHSO3, Na2SO4, Na2SO3, Na2S2O3, K2HPO4, K3PO4, Na2CO3, and 4 M KOH were prepared by dissolving appropriate quantities of these compounds in deionized water. In the case of PEG-6000, the optimum concentration of the PEG-rich phase was also observed to be 50% (w/v) because the solubility of any salt rich phase in the PEG rich phase was minimal at this concentration. Therefore, throughout the experiment, 50% (w/v) of PEG-4000 and PEG-6000 was employed. In many of our earlier experiments, it has been found that lower molecular weight PEGs, such as PEG-400 or PEG-600, are not suitable for metal separation studies. This is because these polymers are like wool balls whose complexing-end becomes difficult to identify.10 Similarly, we have seen that PEG-20000 is also not very effective in separating metal ions.12 Therefore, the extraction studies were performed with 3 mL of various 2 M salt solutions with equal volumes of 50% (w/v) of PEG-4000 as well as PEG-6000 solutions. 0.2 mL of the stock solution containing 97Ru, 95,96Tc and bulk yttrium spiked with 88Y was added to this system and was shaken for 10 min. Then, the system was maintained for 10 min to achieve phase separation before collecting 2 mL of each phase for the γ-spectroscopic studies. Chemical separations were carried out at room temperature. The effect of pH and the efficiency of PEG-6000 over PEG-4000 were also studied.
Back extraction of 97Ru and 95,96Tc into the salt rich phases from the PEG rich phase was carried out using 2 M salt solutions of K2CO3, Na2S2O3 and 4 M KOH. Dialysis was performed using a dialysis membrane sack of suitable length (molecular weight cut-off 1000 Dalton, wet in 0.1% Na-azide) against deionized water on a low speed mechanical shaker to obtain pure NCA 97Ru in an aqueous medium.
Results and discussion
The extraction patterns of 97Ru, 95,96Tc and bulk Y in the PEG-rich phase against different salt-rich phases [Fig. 1] show preferential extraction of 97Ru and 95,96Tc into the PEG phase in all 12 salts-PEG combinations. Ru, along with Tc radionuclides, was extracted to the PEG rich phase without any contamination of bulk yttrium when Na-tartarate, Na-citrate, K2HPO4, K3PO4, KOH, Na2CO3, Na2SO4, Na2SO3 and (NH4)2SO4 were used as the salt solutions. This could be due to the strong complexation of yttrium with citrate, tartarate, HPO4−2, PO4−3, CO3−2, SO4−2 and SO3−2; moreover, bulk Y prefers to stay in the salt-rich phase, whereas the complexing ability of Ru and Tc with these salts was less due to their larger size, and thus they were extracted into the PEG phase. Generally, Ru is present as ruthenate (RuO4−2; calculated ionic radius = ∼180 pm,39) and Tc is present as pertechnate (TcO4−, ionic radius: 206 pm), whereas yttrium is present as free Y3+ (ionic radius: 89 pm,40). Therefore, the preferential extraction of 97Ru and 95,96Tc into the PEG phase could be due to the larger size of Ru and Tc compared to Y. Usually, ions with smaller ionic radii are more solvated and prefer to stay in the salt rich phase, whereas cations with a larger size cations act as hydrophobic molecules, because the entropies of hydration of these ions are positive; thus, they prefer to stay in the PEG-rich phase. However, in three cases, e.g., when Na-malonate, NaHSO4 and Na2S2O3 were used as the salt-rich phase, slight extraction of Y was also observed. The maximum extractions of 97Ru and 95,96Tc in the PEG rich phase were 83% and 96%, respectively, when (NH4)2SO4 was used as the salt-rich phase, without any contamination of bulk yttrium.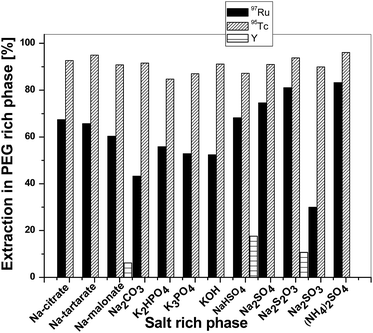 | ||
| Fig. 1 Extraction profiles of 97Ru, 95Tc and bulk Y in the PEG-rich phase against different salt-rich phases at the natural pH of the salts at room temperature. | ||
To study the influence of the molecular weight of PEG on the extraction system, the same experiment was carried out with PEG-6000 against 2 M Na-tartarate, Na-citrate, Na-malonate and K2HPO4 as the salt-rich phases. The results are shown in Fig. 2. It was observed that PEG-6000 has a marginal impact on the extraction patterns of 97Ru, 95,96Tc and bulk Y over PEG-4000. Therefore, all other experiments were carried out with PEG-4000 only.
 | ||
| Fig. 2 Extraction profiles of 97Ru and 95Tc in PEG-6000 against different salt-rich phases at the natural pH of the salts at room temperature (bulk Y was not extracted in any condition). | ||
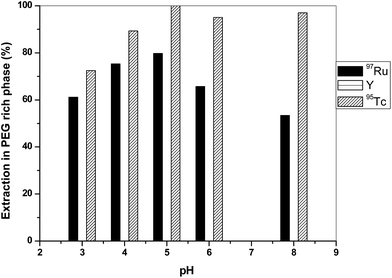
The effect of pH on the extraction of 97Ru, 95,96Tc and bulk Y into the PEG-rich phase was investigated by varying the pH of (NH4)2SO4 and Na-tartarate salt solutions as the salt rich phases (Fig. 3 and 4). The pH of the (NH4)2SO4 and Na-tartarate salt solutions was adjusted using dilute HCl or ammonia solution before mixing with the PEG-rich phase. It has been observed that the extraction patterns of the radionuclides under investigation are almost invariant with changing pH. However, the best separation was obtained at pH 5 when Na-tartarate or (NH4)2SO4 were used as the salt-rich phase. To further improve the chemical yield of 97Ru and 95,96Tc, the relative volumes of PEG-4000 and the salt-rich phase (2 M Na-tartarate or (NH4)2SO4) were varied. In the case of 2 M Na-tartarate, when the volume of PEG phase was doubled compared to the salt rich phase, about 78% 97Ru and 100% 95Tc were extracted into the PEG rich phase. The higher volume of the PEG-rich phase offers more sites for the salting out of 97Ru and 95Tc into the PEG rich phase. In the case of (NH4)2SO4 as the salt-rich phase, the volume of PEG was also increased to improve the chemical yield of 97Ru along with 95,96Tc. However, when the volume of PEG was increased, the extraction of bulk yttrium along with 97Ru and 95,96Tc was observed. The distribution ratios (D) and separation factors (S) of 97Ru, 95Tc and yttrium under various experimental conditions were calculated, and the results are shown in Table 1. Under typical experimental conditions (PEG-4000, 2 M (NH4)2SO4), separation factors (SRu/Y) and (STc/Y) were as high as 4.0 × 103 and 2.0 × 104, respectively.
| Salt-rich phase (2 M) | pH | PEG rich phase | Distribution ratios (D) | Separation factors (S) | ||||
|---|---|---|---|---|---|---|---|---|
| DRu | DY | DTc | SRu/Y | STc/Ru | STc/Y | |||
| Na-citrate | 7 | 4000 | 2.1 | 1.8 × 10−3 | 12.7 | 1.1 × 103 | 6.1 | 7.0 × 103 |
| Na-citrate | 7 | 6000 | 1.4 | 1.2 × 10−3 | 9.8 | 1.2 × 103 | 7.0 | 8.2 × 103 |
| Na-tartarate | 5 | 4000 | 1.9 | 1.1 × 10−3 | 18.8 | 1.7 × 103 | 9.8 | 1.7 × 104 |
| Na-tartarate (3 mL) | 5 | 4000 (6 mL) | 3.5 | 1.4 × 10−3 | 1.0 | 2.5 × 103 | 0.3 | 7.1 × 102 |
| Na-tartarate | 3 | 4000 | 1.6 | 1.4 × 10−3 | 2.6 | 1.1 × 103 | 1.6 | 1.8 × 103 |
| Na-tartarate | 4 | 4000 | 3.0 | 1.4 × 10−3 | 8.3 | 2.1 × 103 | 2.7 | 5.9 × 103 |
| Na-tartarate | 5 | 4000 | 3.9 | 1.4 × 10−3 | 1 | 2.8 × 103 | 0.2 | 0.7 × 103 |
| Na-tartarate | 8 | 4000 | 1.1 | 1.4 × 10−3 | 32.5 | 7.8 × 102 | 29.5 | 23.2 × 103 |
| Na-tartarate | 5 | 6000 | 2.0 | 1.1 × 10−3 | 9.1 | 8.3 × 103 | 4.5 | 8.3 × 103 |
| Na-malonate | 7 | 4000 | 1.5 | 6.5 × 10−2 | 9.8 | 2.3 × 101 | 6.5 | 1.5 × 102 |
| Na-malonate | 7 | 6000 | 1.6 | 1.3 × 10−3 | 5.6 | 2.1 × 103 | 3.5 | 4.3 × 103 |
| Na2CO3 | 11 | 4000 | 0.7 | 1.3 × 10−3 | 10.9 | 5.5 × 102 | 14.3 | 7.9 × 103 |
| K2HPO4 | 8 | 4000 | 1.3 | 9.7 × 10−4 | 5.5 | 1.3 × 103 | 4.4 | 5.7 × 103 |
| K2HPO4 | 8 | 6000 | 1.3 | 9.8 × 10−4 | 5.0 | 1.3 × 103 | 3.8 | 5.1 × 103 |
| K3PO4 | 10 | 4000 | 1.1 | 1.1 × 10−3 | 6.7 | 9.8 × 102 | 9.4 | 5.8 × 103 |
| KOH | 10 | 4000 | 0.1 | 1.3 × 10−3 | 4.3 | 8.9 × 101 | 3.1 | 3.2 × 103 |
| NaHSO4 | 5 | 4000 | 2.1 | 2.1 × 10−1 | 6.8 | 1.0 × 101 | 3.4 | 3.2 × 101 |
| Na2SO4 | 6 | 4000 | 2.9 | 1.3 × 10−3 | 10.1 | 2.1 × 103 | 3.5 | 7.3 × 103 |
| Na2S2O3 | 5 | 4000 | 4.3 | 1.1 × 10−1 | 15.2 | 3.6 × 101 | 20.9 | 1.3 × 102 |
| Na2SO3 | 9 | 4000 | 0.4 | 1.3 × 10−3 | 8.9 | 3.3 × 102 | 4.9 | 6.9 × 104 |
| (NH4)2SO4 | 5 | 4000 | 4.9 | 1.3 × 10−3 | 24.8 | 3.9 × 103 | 6.1 | 1.9 × 104 |
| (NH4)2SO4 | 3 | 4000 | 0.9 | 3.7 × 10−2 | 5.9 | 2.4 × 101 | 6.5 | 1.6 × 102 |
| (NH4)2SO4 | 4 | 4000 | 0.9 | 1.0 × 10−3 | 8.08 | 9.0 × 102 | 8.9 | 8.1 × 103 |
| (NH4)2SO4 | 7 | 4000 | 0.9 | 1.1 × 10−3 | 8.08 | 8.2 × 102 | 8.9 | 7.3 × 103 |
| (NH4)2SO4 (3 mL) | 5 | 4000 (6 mL) | 5.4 | 1.4 × 10−3 | 1.0 | 3.8 × 103 | 0.2 | 7.1 × 102 |
Back extraction of 97Ru from the PEG rich phase
After removing bulk yttrium, back extraction of 97Ru and 95,96Tc into the salt-rich phase from the PEG rich phase was carried out using 2 M of K2CO3, Na2S2O3 and 4 M of KOH as salt rich phases. With 2 M K2CO3 and 4 M KOH, 5% and 14% of 95Tc were also stripped back along with 68% and 81% of 97Ru, respectively, into the salt rich phase. However, with 2 M Na2S2O3, about 93% stripping of 97Ru into the salt-rich phase without any contamination of 95,96Tc was observed [Fig. 5]. Tc may have preferred to stay in the PEG phase due to the larger negative value of the Gibbs free energy of hydration for pertechnate (ΔGhyd: −637 kJ mol−1) compared to ruthenate (ΔGhyd: −307 kJ mol−1).41 | ||
| Fig. 5 Back extraction profiles of NCA 97Ru and co-produced Tc from the PEG rich phase to the salt rich phase. | ||
Dialysis studies of 97Ru containing PEG rich phase
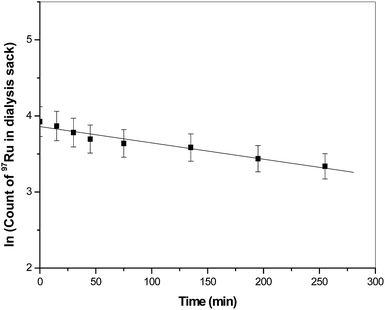
An attempt was made to extract 97Ru into deionized water only. This experiment was carried out with the back-extracted 97Ru fraction in the Na2S2O3 phase (Fig. 5). All the ruthenium was back extracted to the PEG-4000 phase by shaking the salt solution for 10 min with 3 mL PEG-4000. Then, dialysis of the PEG rich phase was carried out again in deionized water. During dialysis, the dissociated 97Ru from the 97Ru-PEG association was continuously removed from the dialysis sack. The percentage of retention of 97Ru in the dialysis sack is shown in Fig. 6. It was observed that after 4 h, about 74% 97Ru was removed from the dialysis sack. 97Ru-PEG association was also plotted against time to measure the half-life of the 97Ru-PEG association [Fig. 7]. The half-life of the association was found to be 5.4 h, which is sufficient for the equilibration process when this complex is present inside the body for therapeutic or diagnostic purposes.Conclusions
An environmental friendly greener method was developed for the separation of Ru and Tc radionuclides from bulk yttrium using a PEG-based aqueous biphasic system. The method is rapid and cost-effective. The synthetic polymer PEG is water-soluble and is not considered to be toxic. However, some applications, e.g., in vivo use of 97Ru, may require these radionuclides to be in an aqueous solution only. Therefore, 97Ru may be obtained in an aqueous medium by dialysis of the PEG phase containing 97Ru. The favorable half-life of 97Ru, its complexing ability, and the simplicity of the separation method we developed without using any toxic chemicals may be useful in future in the field of radiopharmaceuticals and in the clinical application of this radionuclide.Acknowledgements
Authors acknowledge the co-operation of the staff of BARC-TIFR Pelletron facility, Mumbai and TIFR target laboratory, Mumbai for preparing targets and the financial support of SINP-DAE 12th five year plan project TULIP. AD is also thankful to the Council of Scientific and Industrial Research for providing CSIR-RA fellowship.References
- R. D. Rogers, A. H. Bond, J. E. Zhang and P. Horwitz, Sep. Sci. Technol., 1997, 32, 867–882 CrossRef CAS.
- H. Walter, D. E. Brooks and D. Fisher, Partition in aqueous two-phase systems: Theory, methods, uses and applications to biotechnology, Academic press, Orlando, Florida, 1985 Search PubMed.
- R. D. Rogers, J. Zhang, A. H. Bond, C. B. Bauer, M. L. Jezl and D. M. Roden, Solvent Extr. Ion Exch., 1995, 13, 665–688 CrossRef CAS.
- F. Macasek, P. Bartos, P. Gerhart and J. Radioanal, Nucl. Chem., 1998, 229, 87–89 CrossRef CAS.
- R. D. Rogers, A. H. Bond, C. B. Bauer, J. Zhang, S. D. Rein, R. R. Chomko and D. M. Roden, Solvent Extr. Ion Exch., 1995, 13, 689–713 CrossRef CAS.
- R. D. Roger, J. Zhang and S. T. Griffin, Sep. Sci. Technol., 1997, 32, 699–707 CrossRef.
- K. Roy and S. Lahiri, Appl. Radiat. Isot., 2008, 66, 571–576 CrossRef CAS PubMed.
- D. Nayak and S. Lahiri, Appl. Radiat. Isot., 2008, 66, 1793–1798 CrossRef CAS PubMed.
- K. Roy, R. Paul, B. Banerjee and S. Lahiri, Radiochim. Acta, 2009, 97, 637–641 CrossRef CAS.
- S. Lahiri, K. Roy and J. Radioanal, Nucl. Chem., 2009, 281, 531–534 CrossRef CAS.
- B. Dutta, S. Lahiri and B. S. Tomar, Radiochim. Acta, 2013, 101, 19–26 CrossRef CAS.
- A. Datta, M. Maiti, S. Lahiri and J. Radioanal, Nucl. Chem., 2014, 302, 931–937 CrossRef CAS.
- K. Roy and S. Lahiri, Green Chem., 2006, 8, 1063–1066 RSC.
- K. Roy and S. Lahiri, Anal. Chem., 2006, 80, 7504–7507 CrossRef PubMed.
- K. Roy and S. Lahiri, Radiochim. Acta, 2008, 96, 49–53 CrossRef CAS.
- K. Roy and S. Lahiri, Appl. Radiat. Isot., 2009, 67, 1781–1784 CrossRef CAS PubMed.
- S. Lahiri and S. Sarkar, Appl. Radiat. Isot., 2007, 65, 387–391 CrossRef CAS PubMed.
- S. Lahiri and S. Sarkar, Appl. Radiat. Isot., 2007, 65, 309–312 CrossRef CAS PubMed.
- K. Ghosh, M. Maiti, S. Lahiri, V. A. Hussain and J. Radioanal, Nucl. Chem., 2014, 302, 925–930 CrossRef CAS.
- S. C. Srivastava, P. Richards, G. E. Meiken, S. M. Larson and Z. Grunbaum, in Radiopharmaceuticals – Structure – Activity Relationships. Grune & Stratton, ed. R. P. Spencer, New York, 1981 Search PubMed.
- S. C. Srivastava, P. Som, G. Meinken, A. Sewatkar and T. H. Ku, Ruthenium-97 labeled compounds – a new class of radiopharmaceuticals, Brookhaven National Laboratory, Report BNL 24614, 1978 Search PubMed.
- M. C. Lagunas-Solar, M. J. Avila, N. J. Nvarro and P. C. Johnson, Int. J. Appl. Radiat. Isot., 1983, 34, 915–922 CrossRef CAS.
- K. Kofstad, Spallation and fission of silver, Lawrence Berkeley Nat. Lab., Rep. UCRL-2265, 1953 Search PubMed.
- M. S. Uddin, M. Hagiwara, M. Baba, F. Tárkányi and F. Ditroi, Appl. Radiat. Isot., 2005, 62, 533–540 CrossRef CAS PubMed.
- N. G. Zaitseva, V. I. Stegailov, V. A. Khalkin, N. G. Shakun, P. T. Shishlyannikov and K. G. Bukov, Appl. Radiat. Isot., 1996, 47, 145–151 CrossRef CAS.
- N. G. Zaitseva, E. Rurarz, M. Vobecky, K. H. Hwan, K. Nowak, T. Tethal, V. A. Khalkin and L. M. Popinenkova, Radiochim. Acta, 1992, 56, 59–68 CrossRef CAS.
- H. P. Graf, H. Munzel and J. Inorg, Nucl. Chem., 1974, 36, 3647–3657 CrossRef CAS.
- D. Comar and C. Crouzel, Radiochem. Radioanal. Lett., 1976, 27, 307–312 CAS.
- G. Comparetto and S. M. Qaim, Radiochim. Acta, 1980, 27, 177–180 CrossRef CAS.
- N. Ramamaoorthy, M. K. Das, B. R. Sarkar and R. S. Mani, Studies on the production of 97Ru, 38K and 34mCl at the variable energy cyclotron of Bhabha Atomic Research centre, Calcutta, Radiopharmaceuticals and Labelled Compounds, Proc. Int. Conf. 22–26 October 1984, Tokyo, IAEA, Vienna, 1985 Search PubMed.
- P. J. Pao, J. L. Zhou, D. J. Silvester and S. L. Waters, Radiochem. Radioanal. Lett., 1981, 46, 21–26 CAS.
- M. Maiti and S. Lahiri, Radiochim. Acta, 2011, 99, 359–364 CrossRef CAS.
- M. Maiti, Radiochim. Acta, 2013, 101, 437–444 CrossRef CAS.
- S. Lahiri, B. Mukhopadhyay, N. R. Das and J. Radioanal, Nucl. Chem., 1997, 221, 167–171 CrossRef CAS.
- S. Lahiri and B. Mukhopadhyay, Appl. Radiat. Isot., 1997, 48, 925–929 CrossRef CAS.
- M. Maiti and S. Lahiri, Radiochim. Acta, 2015, 103, 7–13 CrossRef CAS.
- B. Dutta, M. Maiti, S. Lahiri and J. Radioanal, Nucl. Chem., 2009, 281, 663–667 CrossRef CAS.
- M. Shibukawa, N. Nakayama, T. Hayashi, D. Shibuya, Y. Endo and S. Kawamura, Anal. Chim. Acta, 2001, 427, 293–300 CrossRef CAS.
- D. Fischer, R. Hoppe, K. M. Mogare and M. Jansen, Z. Naturforsch., B: J. Chem. Sci., 2005, 60, 1113–1117 CAS.
- W. Verweij, CHEAQS PRO: A program for calculating chemical equilibria in aquatic systems, See http://www.home.tiscali.nl/cheaqs/(2005).
- J. P. Icenhower, W. J. Martin, N. P. Qafoku and J. M. Zachara, The geochemistry of technetium: A summary of the behavior of an artificial element in the natural environment PNNL-18139, Richland, WA, 2008 Search PubMed.
Footnote |
| † Present address: Amity Institute of Nuclear Science and Technology, Amity University, Noida-201303, India. |
| This journal is © The Royal Society of Chemistry 2015 |