A renewable resource-derived thixotropic self-assembled supramolecular gel: magnetic stimuli responsive and real-time self-healing behaviour†
Abstract
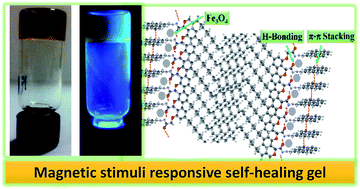
We designed and synthesized a simple fluorescent self-assembled molecular gel from a renewable resource and characterized it well using various techniques. The self-assembly mechanism was studied relative to the molecular structure. We also demonstrate that Fe3O4 nanoparticles were encapsulated in situ into the organogel by a simple process, which generated a magnetic gel with remarkable self-healing features. More importantly, stimuli responsive self-healing properties of the magnetic gel have been characterized by visual inspection, morphological and rheological analyses.


 Please wait while we load your content...
Please wait while we load your content...