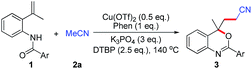
Synthesis of functionalized benzoxazines by copper-catalyzed C(sp3)–H bond functionalization of acetonitrile with olefinic amides†
Xue-Qiang Chu,
Xiao-Ping Xu*,
Hua Meng and
Shun-Jun Ji*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, 215123, China. E-mail: xuxp@suda.edu.cn; shunjun@suda.edu.cn; Fax: +86-512-65880307; Tel: +86-512-65880307
First published on 3rd August 2015
Abstract
A copper-mediated oxycyanomethylation reaction of olefinic amides with acetonitrile was developed for the synthesis of important benzoxazine derivatives. The reactions involve the activation of the C(sp3)–H bond of acetonitrile and radical cyclization processes for the construction of new C–C and C–O bonds.
Benzoxazines have attracted special research interest because they are privileged skeletons existing in a wide variety of pharmaceutical and biological relevant products (Fig. 1).1–4 For example, their versatile utilities as anticonvulsant (I),2 fungicide (II),3 hypolipidemic active agents (III)4 and organic materials.5 As such, considerable efforts have been directed towards the synthesis of this significant heterocycles.6–8 However, some traditional methodologies suffered from prefuctionalization, narrow substrate scope and harsh reaction conditions.6 Recently, the electrophilic cyclization of N-allyl amides with halonium ions7 and even radicals8 have been used to initiate the diverse functionality. Nevertheless, further developing a new and efficient protocol for the construction of benzoxazine derivatives is still urgently demanded.
Direct C–H functionalization of simple alkylnitrile has emerged as an invaluable tool to construct new C–C bond as well as introduce the cyano group in a single operator.9 In the past decades, remarkable advances have been achieved on the catalytic activating α-C–H bond of acetonitrile (low acidity, pKa 31.1 in DMSO).10–13 An elegant example is the cascade palladium-catalyzed 1,2-alkylarylation of N-aryl acrylamides using oxidant PhI(OPiv)2 and additive AgF reported by Liu and co-workers (Scheme 1a).11a Recently, more concise cyanomethyl radical-initiated addition–cyclization by other transition metals such as Cu,11b Ru,11c Fe11d has been documented. Furthermore, Li's,12a Zhu's,12b and our group12c developed a number of new strategies for cyanomethylation with concomitant 1,2-aryl migration of alkenes access to acyclic molecules (Scheme 1b). More recently, Zhu et al. investigated a Cu/DTBP mediated Csp3–C(sp3) and C(sp3)–O bonds formation three-component reaction of alkenes with alkyl nitriles and alcohols (Scheme 1c).13 As a continuation of our interest in the radical pathway transformations,14 we herein describe a copper-catalyzed oxidative C(sp3)–H bond functionalization of acetonitrile with olefinic amides to provide useful benzo[d][1,3]oxazines involving a sequential intermolecular cyanomethylation and intramolecular cyclization (Scheme 1d).
We initiated our work by performing the reaction of N-(2-(prop-1-en-2-yl)phenyl)benzamide 1a with acetonitrile 2a as the model in the present of Cu(OTf)2 (1 equiv.), 1,10-phenanthroline (1 equiv.), di-tert-butyl peroxide (2.5 equiv.) and various base (3 equiv.) at 140 °C for 14 h under air (Table 1, entries 1–6, for more details, see ESI, Table S1†). To our delight, the desired product 3aa was obtained in 67% GC-yield when K3PO4 was used (Table 1, entry 6). The reaction temperature was found to affect the reaction (Table 1, entry 7). Other copper salts such as Cu(OAc)2, Cu(NO3)2, CuI, Cu2O or CuOAc were less effective under otherwise identical conditions (Table 1, entries 8–12). Further reducing the amount of Cu(OTf)2 to 0.5 equiv. increased the GC-yield of product 3aa to 77%, along with an isolated yield of 64% (Table 1, entry 13). Finally, varying different oxidants (K2S2O8, TBPB, DCP, BPO) did not gain better results (Table 1, entries 14–17).
| Entry | Catalyst (equiv.) | Oxidant (2.5 equiv.) | Base (3 equiv.) | GC-yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (2 mL) catalyst (1 equiv.), base (3 equiv.) and oxidant (2.5 equiv.) at 140 °C under air; DTBP = di-tert-butyl peroxide; TBPB = tert-butylperoxybenzoate; DCP = dicumyl peroxide; BPO = benzoyl peroxide.b Yields were determined by GC with an internal standard (biphenyl) as the ratio between the formed products and the initial amount of limiting reactant.c At 120 °C.d Isolated yields. | ||||
| 1 | Cu(OTf)2 (1) | DTBP | K2CO3 | 53 |
| 2 | Cu(OTf)2 (1) | DTBP | Cs2CO3 | Trace |
| 3 | Cu(OTf)2 (1) | DTBP | CsOAc | 55 |
| 4 | Cu(OTf)2 (1) | DTBP | Na2CO3 | 31 |
| 5 | Cu(OTf)2 (1) | DTBP | NaOAc | 20 |
| 6 | Cu(OTf)2 (1) | DTBP | K3PO4 | 67 |
| 7 | Cu(OTf)2 (1) | DTBP | K3PO4 | 50c |
| 8 | Cu(OAc)2 (1) | DTBP | K3PO4 | 55 |
| 9 | CuI (1) | DTBP | K3PO4 | 30 |
| 10 | Cu2O (1) | DTBP | K3PO4 | 59 |
| 11 | CuOAc (1) | DTBP | K3PO4 | 53 |
| 12 | Cu(NO3)2 (1) | DTBP | K3PO4 | 57 |
| 13 | Cu(OTf)2 (0.5) | DTBP | K3PO4 | 77 (64)d |
| 14 | Cu(OTf)2 (0.5) | K2S2O8 | K3PO4 | 0 |
| 15 | Cu(OTf)2 (0.5) | TBPB | K3PO4 | 46 |
| 16 | Cu(OTf)2 (0.5) | DCP | K3PO4 | 63 |
| 17 | Cu(OTf)2 (0.5) | BPO | K3PO4 | 23 |
With the optimal conditions in hand, the generality and substrate scope of olefinic amides 1 was firstly examined. As shown in Table 2, this copper-catalyzed oxycyanomethylation tolerated a wide range of functional groups. Benzamides bearing electron-donating (4-OMe, 4-Et, 3-Me, 3-NMe2) or electron-withdrawing (4-SO2Me, 4-NO2) groups on the benzoyl moiety were effectively converted to the corresponding products in moderate yields (Table 2, 3ba–ea and 3ja, 3ka). Gratifyingly, substrates with halogen atoms such as F, Cl, Br and I could be employed in this process to give benzoxazines 3fa–3ia in 49–64% (Table 2, 3fa–3ia). Although the steric effect of ortho-substituent aromatic ring reduced the cyclization reactivity, the targeted products 3ia (2-Cl), 3ma (2-NO2) and 3na (2,4-NO2) were still obtained in spite of lower yields (Table 2, 3ia–3na). Additionally, 2-naphthoic and heterocyclic derivatives also proved to be suitable for this transformation, affording the desired products with satisfactory yields (Table 2, 3oa, 3pa).
| a Under the optimal conditions; yields of isolated products. |
|---|
 |
During the course of our studies, it was found that phenyl ring at α-position of alkene gave 3qa in yield (37%) lower than which containing the methyl group, which may be attributed to the steric hindrance effect (Table 3, 3qa). Aliphatic 1r and 1s could also be successfully incorporated into the skeleton of benzoxazines (Table 3, 3ra, 3sa). Cinnamamide 1u produced the alkylated product 3ua in 24% yield (Table 3, 3ua). However, no product was detected when 1t was used as a candidate. Moreover, other α-substituted alkylnitriles 2b–2e (n-Pr, Ph, OMe, Br) failed to participate in this catalytic system (Table 3, 3ab–3ae).
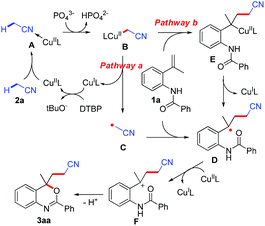
A radical-trapping experiment was carried out to understand the details of the mechanism. The reaction was completely suppressed in the presence of 2,2,6,6-tetramethylpiperidine N-oxide (TEMPO), only TEMPO adduct 4 and 5 was observed under the standard conditions (determined by LC-MS analysis, Fig. S1 in ESI,† homolysis of peroxide with the assistance of copper followed by β-cleavage of the butyloxyl radical would form the methyl radical), which indicated that a free radical process would be involved (Scheme 2). On the basis of the above results and previous reports,9–13 a possible mechanism has been shown in Scheme 3. Firstly, coordination of acetonitrile to a ligated copper(II) complex followed deprotonation by K3PO4 would generate cyanomethyl copper species A.15 Next, organocopper A might decompose to give cyanomethyl radical C, which underwent addition with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of olefinic amides 1, affording an alkyl radical D (Scheme 3, pathway a). Alternatively, direct carbocupration of alkene with A followed by homolytic cleavage afforded the radical intermediate D (Scheme 3, pathway b).10d,e,13 Finally, the desired benzoxazine 3 was delivered via further oxidation and intermolecular cyclization (trapped by the carbonyl group of amide). Oxidation of CuI species by DTBP would regenerate the CuII complex.16
C bond of olefinic amides 1, affording an alkyl radical D (Scheme 3, pathway a). Alternatively, direct carbocupration of alkene with A followed by homolytic cleavage afforded the radical intermediate D (Scheme 3, pathway b).10d,e,13 Finally, the desired benzoxazine 3 was delivered via further oxidation and intermolecular cyclization (trapped by the carbonyl group of amide). Oxidation of CuI species by DTBP would regenerate the CuII complex.16
In summary, we have developed a Cu-catalyzed oxycyanomethylation reaction of olefinic amides with acetonitrile. The protocol provided a straight forward route to important benzoxazine derivatives via C(sp3)–H functionalization of simple nitrile and difunctionalization of unactivated alkenes. Future work will be focused on further exploration of the reaction mechanism.
Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (no. 21172162, 21372174), the Young National Natural Science Foundation of China (no. 21202113), the Ph.D. Programs Foundation of Ministry of Education of China (2013201130004), the Research Grant from the Innovation Project for Graduate Student of Jiangsu Province (KYZZ15_0322), PAPD, and Soochow University for financial support.Notes and references
- (a) A. Krantz, R. W. Spencer, T. F. Tam, T. J. Liak, L. J. Copp, E. M. Thomas and S. P. Rafferty, J. Med. Chem., 1990, 33, 464 CrossRef CAS; (b) S. J. Hays, B. W. Caprathe, J. L. Gilmore, N. Amin, M. R. Emmerling, W. Michael, R. Nadimpalli, R. Nath, K. J. Raser, D. Stafford, D. Watson, K. Wang and J. C. Jaen, J. Med. Chem., 1998, 41, 1060 CrossRef CAS PubMed; (c) P. Zhang, T. A. Terefenko, A. Fensome, Z. Zhang, Y. Zhu, J. Cohen, R. Winneker, J. Wrobel and J. Yardley, Bioorg. Med. Chem. Lett., 2002, 12, 787 CrossRef CAS; (d) J. W. Kobzina, US4030906, 1977; (e) T. Tsubuki, T. Sagawa, K. Funada and I. Araya, WO2011036895, 2011.
- H. Kuch, K. Schmitt, G. Seidl and I. Hoffmann, US 3725404, 1973.
- H. Sugiyama, K. Hosoda, Y. Kumagai, M. Takeuchi and M. Okada, US 4596801, 1986.
- G. Fenton, C. G. Newto, B. M. Wyman, P. Bagge, D. I. Dron, D. Riddell and G. D. Jones, J. Med. Chem., 1989, 32, 265 CrossRef CAS.
- (a) Y. L. Liu, C. W. Hsu and C. I. Chou, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1007 CrossRef CAS PubMed; (b) Z. Wang, Q. Ran, R. Zhu and Y. Gu, RSC Adv., 2013, 3, 1350 RSC; (c) H. Wang, J. Wang, X. He, T. Feng, N. Ramdani, M. Luan, W. Liu and X. Xu, RSC Adv., 2014, 4, 64798 RSC.
- Recent examples, see: (a) Y. Li, Z. Li, T. Xiong, Q. Zhang and X. Zhang, Org. Lett., 2011, 15, 1098 Search PubMed; (b) W.-C. Lee, H.-C. Shen, W.-P. Hu, W.-S. Lo, C. Murali, J. K. Vandavasi and J.-J. Wang, Adv. Synth. Catal., 2012, 354, 2218 CrossRef CAS PubMed; (c) Q. Liu, P. Chen and G. Liu, ACS Catal., 2013, 3, 178 CrossRef CAS; (d) B. V. S. Reddy, R. A. Babu, M. R. Reddy, B. J. M. Reddy and B. Sridhar, RSC Adv., 2014, 4, 44629 RSC; (e) S. Munusamy, S. Venkatesan and K. I. Sathiyanarayanan, Tetrahedron Lett., 2015, 56, 203 CrossRef CAS PubMed; (f) E. Cahard, H. P. J. Male, M. Tissot and M. J. Gaunt, J. Am. Chem. Soc., 2015, 137, 7986 CrossRef CAS PubMed.
- (a) K. Okuma, T. Yasuda, I. Takeshita, K. Shioji and Y. Yokomori, Tetrahedron, 2007, 63, 8250 CrossRef CAS PubMed; (b) A. Jaganathan, A. Garzan, D. C. Whitehead, R. J. Staples and B. Borhan, Angew. Chem., Int. Ed., 2011, 50, 2593 CrossRef CAS PubMed; (c) E. Cahard, N. Bremeyer and M. J. Gaunt, Angew. Chem., Int. Ed., 2013, 52, 9284 CrossRef CAS PubMed; (d) Q. Yin and S.-L. You, Org. Lett., 2014, 16, 2426 CrossRef CAS PubMed.
- (a) Q.-H. Deng, J.-R. Chen, Q. Wei, Q.-Q. Zhao, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2015, 51, 3537 RSC; (b) H. Yang, X.-H. Duan, J.-F. Zhao and L.-N. Guo, Org. Lett., 2015, 17, 1998 CrossRef CAS PubMed.
- Selected reviews, see: (a) G. C. Lloyd-Jones, Angew. Chem., Int. Ed., 2002, 41, 953 CrossRef CAS; (b) D. A. Culkin and J. F. Hartwig, Acc. Chem. Res., 2003, 36, 234 CrossRef CAS PubMed; (c) C. C. C. Johansson and T. J. Colacot, Angew. Chem., Int. Ed., 2010, 49, 676 CrossRef CAS PubMed; (d) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082 CrossRef CAS PubMed.
- Recent examples, see: (a) Y. Kawato, N. Kumagai and M. Shibasaki, Chem. Commun., 2013, 49, 11227 RSC; (b) J. Shen, D. Yang, Y. Liu, S. Qin, J. Zhang, J. Sun, C. Liu, C. Liu, X. Zhao, C. Chu and R. Liu, Org. Lett., 2014, 16, 350 CrossRef CAS PubMed; (c) S. B. Lang, T. M. Locascio and J. A. Tunge, Org. Lett., 2014, 16, 4308 CrossRef CAS PubMed; (d) A. Bunescu, Q. Wang and J. Zhu, Org. Lett., 2015, 17, 1890 CrossRef CAS PubMed; (e) Z. Li, Y. Xiao and Z.-Q. Liu, Chem. Commun., 2015, 51, 9969 RSC.
- (a) T. Wu, X. Mu and G. Liu, Angew. Chem., Int. Ed., 2011, 50, 12578 CrossRef CAS PubMed; (b) J. Li, Z. Wang, N. Wu, G. Gao and J. You, Chem. Commun., 2014, 50, 15049 RSC; (c) J.-L. Zhang, Y. Liu, R.-J. Song, G.-F. Jiang and J.-H. Li, Synlett, 2014, 1031 Search PubMed; (d) C. Pan, H. Zhang and C. Zhu, Org. Biomol. Chem., 2015, 13, 361 RSC.
- (a) Y. Li, B. Liu, H.-B. Li, Q. Wang and J.-H. Li, Chem. Commun., 2015, 51, 1024 RSC; (b) A. Bunescu, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 3132 CrossRef CAS PubMed; (c) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Org. Chem. Front., 2015, 2, 216 RSC.
- C. Chatalova-Sazepin, Q. Wang, G. M. Sammis and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 5443 CrossRef CAS PubMed.
- (a) J.-J. Cao, T.-H. Zhu, S.-Y. Wang, Z.-Y. Gu, X. Wang and S.-J. Ji, Chem. Commun., 2014, 50, 6439 RSC; (b) X.-Q. Chu, Y. Zi, H. Meng, X.-P. Xu and S.-J. Ji, Chem. Commun., 2014, 50, 7642 RSC; (c) J.-J. Cao, X. Wang, S.-Y. Wang and S.-J. Ji, Chem. Commun., 2014, 50, 12892 RSC; (d) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Chem. Commun., 2014, 50, 9718 RSC; (e) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Chem.–Eur. J., 2014, 20, 17198 CrossRef CAS PubMed.
- Organocopper species, see: (a) M. Purzycki, W. Liu, G. Hilmersson and F. F. Fleming, Chem. Commun., 2013, 49, 4700 RSC; (b) E. J. Corey and I. Kuwajima, Tetrahedron Lett., 1972, 13, 487 CrossRef.
- Recent examples of copper–DTBP mediated reactions, see: (a) A. K. Yadav and L. S. Yadav, Org. Biomol. Chem., 2015, 13, 2606 RSC; (b) P. K. Chikkade, Y. Kuninobu and M. Kanai, Chem. Sci., 2015, 6, 3195 RSC; (c) B. D. Barve, Y.-C. Wu, M. El-Shazly, M. Korinek, Y.-B. Cheng, J.-J. Wang and F.-R. Chang, Tetrahedron, 2015, 71, 2290 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14729h |
| This journal is © The Royal Society of Chemistry 2015 |