Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity
Kaïs Rtibi*ab,
Mohamed Amine Jabribc,
Slimen Selmib,
Abdelaziz Soulia,
Hichem Sebaibc,
Jamel El-Bennad,
Mohamed Amria and
Lamjed Marzoukib
aLaboratoire de Neurophysiologie Fonctionnelle et Pathologies, Département des Sciences Biologiques, Faculté des Sciences de Tunis, Campus Universitaire El Manar II-2092, Tunis, Tunisia. E-mail: rtibikais@yahoo.fr; Fax: +216 72 590 566; Tel: +216 97 479 135
bLaboratoire de Physiologie Fonctionnelle et Valorisation des Bio-Resources, Institut Supérieur de Biotechnologie de Béja, Université de Jendouba, Avenue Habib Bourguiba, B.P., 382-9000 Béja, Tunisia
cLaboratoire de Physiologie Intégrée, Faculté des Sciences de Bizerte, 7021 Zarzouna, Tunisia
dINSERM U773 Centre de Recherche Biomédicale, Faculté de Médecine X. Bichat, 75018 Paris, France
First published on 8th September 2015
Abstract
Natural antioxidants such as phenolic compounds protect cells against the damaging effects of reactive oxygen species (ROS). In this study, we investigated the effect of carob pods aqueous extract (CPAE) on the reactive oxygen species (ROS) production by human neutrophils, myeloperoxidase (MPO) activity and expression as well as lactoferrin and NADPH oxidase phosphorylation. Neutrophils were isolated from whole human blood using the ficoll–dextran method and ROS generation was measured by luminol-amplified chemiluminescence. Superoxide anion generation was detected by chemiluminescence using the lucigenin method. H2O2 was detected by the chemiluminescence assay. MPO activity was measured by the tetramethylbenzidine oxidation method. Western blotting analysis was used to determine the MPO and lactoferrin as well as P47phox–Ser-328 phosphorylation. The use of HPLC technique revealed the identification of many phenolic compounds in carob pods with pyrogallol as the main compound in the pulp and tannic acid in the seeds. We also found that CPAE inhibits luminol-amplified chemiluminescence in human neutrophils stimulated by PMA and that it is able to scavenge superoxide anion and hydrogen peroxide. Carob extract significantly reduces MPO activity and expression. More importantly, CPAE inhibits PMA-induced p47phox phosphorylation on Ser328 as well as lactoferrin release by neutrophils in a concentration-dependent manner. The effects are generally more marked for the seeds compared to the pulp. In conclusion, we suggest in the present study that Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS production.
1. Introduction
The immune system is especially vulnerable to oxidative damage because many immune cells, such as neutrophils, produce reactive oxygen and nitrogen species (ROS/RNS) as part of the body's defense mechanisms to destroy invading pathogens.1 They release reactive oxygen species and cytokines outside the cells to kill extracellular bacteria and recruit additional leukocytes to the region of infection or inflammation.2 Therefore, neutrophils represent the body's primary line of defense against invading pathogens and inflammation.3 Neutrophil granules are classified into three distinct subsets based on the presence of characteristic granule proteins: primary (azurophil) granules (myeloperoxidase (MPO)), secondary (specific) granules (lactoferrin), and tertiary (gelatinase) granules (gelatinase). Excessive neutrophil degranulation is a common feature of many inflammatory disorders such as severe asphyxic episodes of asthma, acute lung injury, rheumatoid arthritis, and septic shock.1The generation of microbicidal oxidants by neutrophils leads to the activation of a multiprotein enzyme complex known as the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is responsible for transferring electrons from NADPH to O2, resulting in the formation of a superoxide anion. This multi-component enzyme system is composed of cytosolic proteins (p47phox, p67phox, p40phox, and rac1/2) and membrane proteins (p22phox and gp91phox, which form cytochrome b558), which assemble at membrane sites upon cell activation. NADPH oxidase activation is tightly regulated because of potential damage to the surrounding tissues.4–6 P47phox phosphorylation on several serines plays a pivotal role in oxidase activation in intact cells.7,8
The response of neutrophils to several biologically active substances, e.g. to arachidonic acid, phorbolmyristate acetate (PMA), N-formyl-methionylleucylphenylalanine (FMLP) or to calcium ionophore A23187, mimics the inflammation-induced burst of neutrophils as well as the production and release of superoxide anion radical (O2˙−). Subsequently, O2˙− may initiate the formation of derived ROS: hydrogen peroxide (H2O2), singlet oxygen, hydroxyl radical (OH˙) and hypochlorous acid (HOCl).9–11 Therefore, depending on the factors of concentration, duration, and localization of their production, ROS either play a beneficial role by participating in tissue homeostasis or when inappropriate or excessive production occurs, can directly damage the surrounding tissues and participate in inflammatory disorders.12 In addition to the ROS production, the cytoplasmic granules of the neutrophils discharge hydrolytic and proteolytic enzymes and myeloperoxidase (MPO; EC 1.11.1.7) that is a hemic enzyme specific of azurophilic granules. MPO is unique because it has a dual activity of peroxidase and chlorination reaction.
In this perspective, the use of natural polyphenols is promising. These compounds have antioxidant, anti-inflammatory and antitumor activities.13 Antioxidant activities of plant polyphenols have been claimed to have beneficial health functions for retarding aging and preventing cancer and cardiovascular diseases.14 In vitro, Gismondi et al.15 recently showed some antioxidant capabilities of the African medicinal plant as well as the capabilities of cell cycle arrest and differentiation in B16F10 melanoma cells. With the same cell model, Forni et al.16 suggested an antineoplastic activity of strawberry (Fragaria × ananassa Duch.) crude extracts. For these reasons, the natural antioxidants and anti-inflammatory compounds have recently become a major area of research.
The carob tree (Ceratonia siliqua L., Leguminosae family) has been widely cultivated for years in Mediterranean countries, including Tunisia.17 The carob fruit, a brown pod 10–25 cm in length, contains two major components: pulp and the seeds.18 This plant is rich in bioactive molecules such as phenolic compounds.19 Recently, we have shown that the water extract of carob pods presents a good in vitro free-radical scavenging ability,20 higher than many other foods rich in phenolic compounds such as blueberries and grapes.21,22
The aim of the present study was to examine the effect of carob pods aqueous extract (CPAE) on ROS production by human neutrophils as well as its effect on MPO, lactoferrin expression, and P47phox–Ser-328 phosphorylation.
2. Materials and methods
2.1. Chemicals and reagents
Luminol, PMA, lucigenin, and HRPO were from Sigma-Aldrich (Saint-Quentin Fallavier, France). HBSS and HEPES were from Gibco. Ficoll and dextran T500 were from GE Healthcare. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE) and western blotting reagents were purchased from Bio-Rad (Richmond, CA, USA). Polyclonal rabbit anti-p47phox and antibodies were previously described.23 The rabbit polyclonal antibodies against anti-phospho-Ser328–p47phox, lactoferrin and MPO have been produced using specific peptides made by PolyPeptide Laboratories (Strasbourg, France).24 The different solutions were diluted in phosphate-buffered saline (PBS) immediately before use.2.2. Ethics statement
The necessary permits for the field studies and collection of carob pod samples were obtained from the Ministry of Agriculture in Tunisia and identified by Mrs Mouhiba Ben-Naceur, professor of taxonomy in the Higher Institute of Biotechnology of Béja-Tunisia. The voucher specimens have been deposited with the herbarium of the Higher Institute of Biotechnology of Beja and also in the Department of Biological Sciences, Faculty of Science of Tunis.The Clinical Research Committee at the Xavier Bichat hospital (Paris, France) approved all protocols, and we obtained written consent from each blood donor.
2.3. Preparation of carob extracts
The mature carob pods were collected from the region of Tabarka (North-West of Tunisia) during October 2013. Briefly, the seeds were rigorously separated from the pulp, dried in an incubator at 50 °C during 72 hours and powdered in an electric blender (Moulinex Ovatio 2, FR). Seeds and pulp powder were dissolved in double distilled water and filtered through a colander (0.5 mm mesh size). Finally, the lyophilized extracts of carob pulp and seeds were immediately used.2.4. Hydrolysis and analysis of phenolic compounds using RP-HPLC
Dried samples from pulp and seeds were hydrolyzed according to the slightly modified method of Proestos et al.25 The acidic hydrolysis was used to release the aglycones to simplify the identification process since the free forms of phenolic compounds are rarely present in plants and they occur as esters, glycosides, or bound to the cell wall.26 Twenty milliliters of methanol were added to 0.5 g of a dried sample. Then, 10 mL of 1 M HCl was added. The mixture was stirred carefully and sonicated for 15 min and refluxed in a water bath at 90 °C for 2 h. The obtained mixture was injected into a HPLC. The separation was carried out on a 250 mm × 4.6 mm, 5 μm Hypersil ODS C18 reversed phase column at ambient temperature. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2% formic acid (solvent B). The flow rate was maintained at 0.7 mL min−1. The gradient program was as follows: 35% A/65% B, 0–6 min; 60% A/40% B, 6–9 min; 80% A/20% B, 9–14 min and 100% A, 14–25 min. The injection volume was 20 μL, and peaks were monitored at 280 nm. Samples were filtered through a 0.45 μm membrane filter before injection. Phenolic compounds were identified by congruent retention times compared with standards. Analyses were performed in triplicate.2.5. Fractionation of human neutrophils
Venous blood was collected from healthy adult volunteers and neutrophils were isolated by dextran sedimentation and density gradient centrifugation as previously described.27 Erythrocytes were removed by hypotonic lysis. After the isolation, the cells were resuspended in an appropriate medium such as Hank's balanced salt solution (HBSS). The cells were counted and their viability was determined with the trypan blue exclusion method.2.6. Measurement of ROS production by chemiluminescence
Isolated cells were resuspended in HBSS at a concentration of 1 million per mL. Cell suspensions (5 × 105) in 0.5 mL of HBSS containing 10 μM luminol in the presence or absence of CPAE were added after dilution using a micropipette, preheated at 37 °C in the thermostated chamber of a luminometer (Berthold-Biolumat LB937) and allowed to stabilize. After a baseline reading, cells were stimulated with PMA (100 ng mL−1). Changes in chemiluminescence were measured over a 30 min period.282.7. Preparation of azurophilic granules and measurement of MPO activity
Neutrophils were lysed by nitrogen cavitation and the granule fraction was purified by Percol gradient centrifugation.29 The granules were sonicated in 0.2 CTAB and MPO activity was assessed using the H2O2-dependent tetramethylbenzidine (TMB) oxidation assay at 650 nm.30 MPO content was also measured by western blotting analysis and ELISA.2.8. H2O2 chemiluminescence assay
The chemiluminescence luminol-based method has been used for the determination of H2O2, which is based on a reaction with H2O2 catalyzed by horseradish peroxidase (HRP).312.9. Lucigenin-dependent chemiluminescence assay for O2˙−
The accumulation of O2˙− is measured by the chemiluminescence of lucigenin.322.10. Electrophoresis and immunoblotting
Immunoprecipitated proteins were separated on SDS-polyacrylamide gels using standard techniques.33 Proteins were transferred to nitrocellulose, and the phosphorylation of p47-phox, P47phox–Ser-328, MPO and lactoferrin were monitored by immunoblotting using monoclonal or polyclonal antibodies.2.11. Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) and were expressed as means ± standard error of the mean (SEM). The data are representative of five independent experiments. All statistical tests were two-tailed, and a p value of 0.05 or less was considered significant.3. Results
3.1. Identification and quantification of phenolic compounds by HPLC analysis
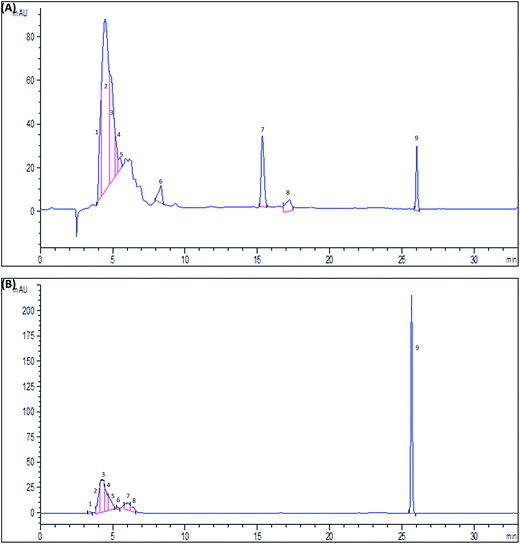
The use of HPLC technique (Fig. 1) revealed the identification of various phenolic compounds, as shown in Table 1. The principal compounds are as follows: pyrogallol (48.02% ± 3.55% and 9.12% ± 2.04%), catechin (19.10% ± 2.11% and 6.25% ± 1.10%) and tannic acid (9.01% ± 1.40% and 19.03% ± 2.13%), respectively, in pulp and seed compartments.| % of phenolic compounds | ||
|---|---|---|
| Pulp | Seeds | |
| Gallic acid | 3.10 ± 0.62 | 0.80 ± 0.30 |
| Tannic acid | 9.01 ± 1.40 | 19.03 ± 2.13 |
| Caffeic acid | 1.71 ± 0.41 | 00 |
| Ferulic acid | 3.10 ± 0.72 | 00 |
| Vanillic acid | 00 | 2.71 ± 0.64 |
| Pyrogallol | 48.02 ± 3.55 | 9.12 ± 2.04 |
| Catechin | 19.10 ± 2.11 | 6.25 ± 1.10 |
3.2. CPAE inhibits luminol-amplified chemiluminescence in human neutrophils stimulated by PMA
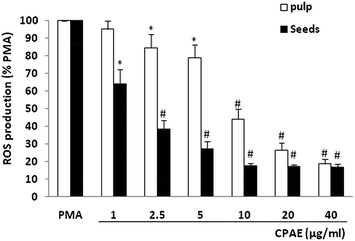
To study the effect of carob extract on human neutrophil ROS production, cells were incubated with different CPAE concentrations and ROS were detected by luminol-amplified chemiluminescence. Results show that CPAE (pulp and seeds) inhibited luminol-amplified chemiluminescence on neutrophils stimulated by PMA in a concentration-dependent manner (Fig. 2). The CI50 values calculated from the graph demonstrate that the inhibition of ROS production by neutrophils was more marked for the seeds (CI50 = 3.4 μg mL−1) compared to the pulp (CI50 = 12.5 μg mL−1).3.3. The effect of CPAE on neutrophil superoxide anion production
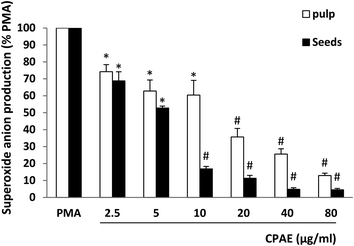
To investigate the effect of aqueous extract of carob pods on NADPH oxidase activity, we studied its effect on superoxide anion production using a lucigenin-dependent chemiluminescence assay. As shown in Fig. 3, CPAE of pulp and seeds significantly inhibit PMA-induced superoxide anion production in a concentration-dependent manner. The CI50 values calculated from the graph clearly show that the effect is more marked for the seeds (CI50 = 5.3 μg mL−1) compared to the pulp (CI50 = 6.7 μg mL−1).3.4. Effect of CPAE on horseradish peroxidase-induced H2O2 production
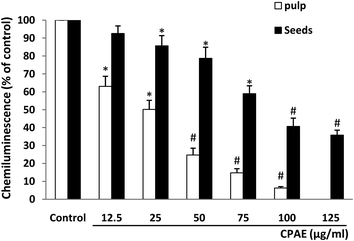
We further examined the effect of aqueous extract of carob pods on horseradish peroxidase (HRP)-induced H2O2 production. Results show that CPAE (pulp and seeds) significantly and dose-dependently decreased the luminol-amplified chemiluminescence (Fig. 4). Contrary to all ROS and superoxide anion production, the effect on H2O2 is more marked for the pulp (CI50 = 25 μg mL−1) compared to the seeds (CI50 = 87.4 μg mL−1).3.5. Effect of CPAE on MPO activity and expression from azurophilic granules
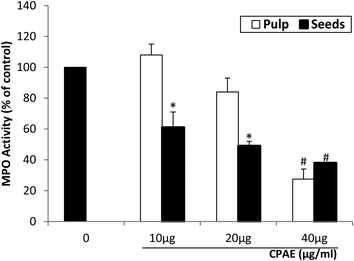
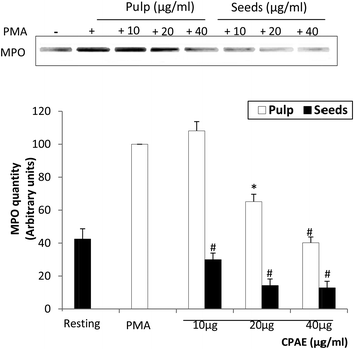
We also tested the effect of CPAE on MPO activity and expression. As shown in Fig. 5, CPAE inhibited the MPO activity in a concentration-dependent manner. Moreover, the western blot analysis shows that PMA administration clearly stimulated the expression of MPO, whereas the CPAE co-treatment significantly abolished PMA-induced MPO expression in a dose-dependent manner (60% and 85%, respectively, for the pulp and the seeds with the high concentration of CPAE) (Fig. 6). In both cases, the effect is more marked for the seeds compared to the pulp.3.6. CPAE inhibits PMA-induced p47phox phosphorylation on Ser328
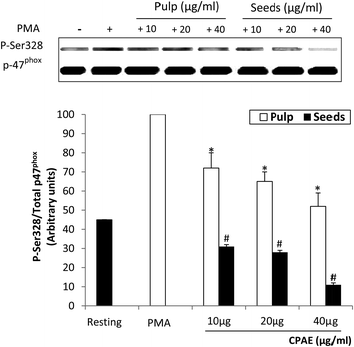
Using a specific antibody directed against this phosphorylated site, we further analyzed the effect of CPAE on intracellular protein phosphorylation. Results showed that CPAE inhibited PMA-induced p47phox phosphorylation on Ser328 in a dose-dependent manner (50% and 90%, respectively, for the pulp and the seeds with the high concentration of CPAE). Western blot analysis also showed the same amount of proteins for all tested conditions (Fig. 7).3.7. CPAE inhibits PMA-induced lactoferrin release by neutrophils
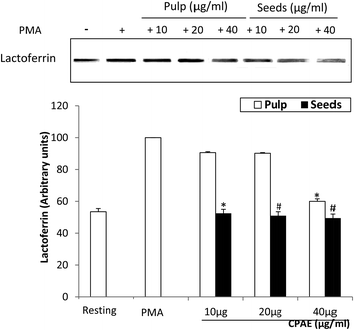
We further examined the effect of aqueous extract of carob pods (pulp and seeds) on the release of lactoferrin present in the secondary granules of neutrophil granulocytes. As shown in Fig. 8, the use of western blot analysis shows that CPAE co-treatment inhibited the PMA-induced lactoferrin release in a concentration-dependent manner, indicating an inhibition of neutrophil degranulation. The effect is more marked for the seeds (50%) compared to the pulp (40%) with the high carob extract concentration.4. Discussion
In the present study, we investigated the effect of carob pod (Ceratonia siliqua L.) aqueous extracts on human neutrophils myeloperoxidase and on in vitro ROS-scavenging activity.Our phytochemical study firstly revealed that CPAE is rich in total polyphenols, flavonoids and condensed tannins. In addition, using the ABTS radical-scavenging assay, we found that CPAE of pulp and seeds exhibits a high scavenging capacity, albeit lesser than ascorbic acid, which was used as a reference molecule.20 The use of the HPLC technique revealed the identification of phenolic compounds in carob pods with pyrogallol and catechin as main compounds in the pulp and tannic acid in the seeds. However, previous studies have reported that these molecules are well known for their antioxidant properties.34,35 The variability in chemical composition as well as in phenolic compounds content can be attributed to the climatic conditions as well as the solvent and the mode of extraction.20 Importantly, we showed that the aqueous carob pods extract (pulp and seeds) inhibits ROS, superoxide anion and H2O2 production in a concentration-dependent manner.
We also examined the effect of CPAE and its ability to inhibit the phosphorylation of p47phox–Ser-328. We found that p47phox phosphorylation on p47phox–Ser-328 phosphorylation was modulated by CPAE, which induces the inhibition of NADPH oxidase activity. In addition, we showed that CPAE inhibits the MPO activity in a concentration-dependent manner. MPO inhibition by carob extracts has therefore the ability to reduce the production of hypochlorous acid (HOCl) from H2O2 and could attenuate the inflammatory reactions.
More importantly, our present results showed that incubation of neutrophils with CPAE caused a significant inhibition of lactoferrin release from stimulated human cells induced by PMA stimulation in a dose dependent manner. Lactoferrin (LF), an iron-binding protein found in specific granules of neutrophils, enhances OH˙ generation by human neutrophils, by a xanthine oxidase system that generates oxygen radicals.36 In addition, to enhance OH˙ production, previous reports have suggested that LF may play a role in neutrophils bactericidal activity. Moreover, Wang-Iverson et al.37 have shown a defect in bactericidal activity at high bacteria-to-cell ratios with neutrophils depleted of specific granules and LF by pretreatment with PMA.
These results also corroborate those of Corsi et al.,38 who identified mainly gallic acid, (−)-epigallocatechin-3-gallate (EGCG) and (−)-epicatechin-3-gallate (ECG) in an infusion of carob pods with boiling water. Other investigations demonstrated that carob pods contain a large spectrum of hydrolysable tannins such as gallotannins and ellagitannins, which explain the high antioxidant capacity of this fruit.39–41 However, Temiz et al.42 recently concluded that carob extract showed a hepatoprotective effect and antioxidant capacity in rats with ethanol toxicity, probably acting by promoting the antioxidative defense systems.
Several studies provide an overview of the currently available NADPH oxidase inhibitors derived from natural extracts such as polyphenols. Indeed, resveratrol is a naturally occurring polyphenol, which has vasoprotective effects in diabetic animal models and inhibits high glucose (HG)-induced oxidative stress in endothelial cells. It has been reported that HG induces endothelial cells apoptosis through a NF-ΚB/NADPH oxidase/ROS pathway, which was inhibited by resveratrol.43,44
A previous study using Ginkgo biloba extract was tested for its effect on ROS release during human neutrophils stimulation by a soluble agonist.45 However, this extract slowed down the oxygen consumption (respiratory burst) of the stimulated cells by its inhibitory action on NADPH oxidase. Moreover, Ginkgo biloba also had a higher free radical scavenging activity.46 Another study used tea polyphenols to demonstrate a beneficial effect against the development of tumor promotion and progression through both ROS scavenging and NADPH oxidase.47 On the other hand, Pastene et al.48 studied the effects of a standardized extract of apple peel (60% of total polyphenols, 58% of flavonoids) with regard to the intra- and extra-cellular production of ROS in human neutrophils stimulated by Helicobacter pylori, PMA, or fMLP. Indeed, apple-peel extract inhibited the respiratory burst of neutrophils induced by all three activators in a concentration-dependent manner. A better understanding of phenomena involved in the regulation of NADPH oxidase could help to develop novel therapeutic agents for inflammatory diseases, involving abnormal superoxide production by neutrophils. However, a therapeutic goal could lead to lowering the oxidant activity of stimulated neutrophils and the MPO activity. Therefore, the novel strategy of anti-inflammatory and antioxidant therapy is based upon pharmacological agents capable of enhancing the resolution of inflammation and oxidative stress.49–51
5. Conclusion
In conclusion, our data clearly demonstrate that the aqueous extracts of carob pods (pulp and seeds) inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity owing to, in part, their antioxidant properties.Declaration of interest
The authors alone are responsible for the content of this paper.Acknowledgements
The authors would like to thank all the members of U1149, Center for Research on Inflammation, Paris, France, for assistance and helpful discussion. Financial support of the INSERM, the Tunisian Ministry of Higher Education and the Scientific Research are gratefully acknowledged. Financial disclosures: none declared.References
- D. P. Marin, A. P. Bolin, C. MacedoRde, S. C. Sampaio and R. Otton, ROS production in neutrophils from alloxan-induced diabetic rats treated in vivo with astaxanthin, Int. Immunopharmacol., 2011, 11, 103–109 CrossRef CAS PubMed.
- L. Paige, Mechanisms of Degranulation in Neutrophils, Allergy, Asthma, Clin. Immunol., 2006, 2, 98–108 CrossRef PubMed.
- J. Viera, P. Tomas, H. Juraj, N. Radomir and D. Katarina, Decreased activity and accelerated apoptosis of neutrophils in the presence of natural polyphenols, Interdiscip. Toxicol., 2012, 5, 59–64 Search PubMed.
- K. Bedard and K. H. Krause, The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology, Physiol. Rev., 2007, 87, 245–313 CrossRef CAS PubMed.
- J. El-Benna, P. M. Dang, M. A. Gougerot-Pocidalo and C. Elbim, Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses, Arch. Immunol. Ther. Exp., 2005, 53, 199–206 CAS.
- K. H. Krause and K. Bedard, NOX enzymes in immuno-inflammatory pathologies, Semin. Immunopathol., 2008, 30, 193–194 CrossRef PubMed.
- J. El Benna, L. P. Faust and B. M. Babior, The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases, J. Biol. Chem., 1994, 269, 23431–23436 CAS.
- L. R. Faust, J. El-Benna, B. M. Babior and S. J. Chanock, The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site directed mutagenesis, J. Clin. Invest., 1995, 96, 1499–1505 CrossRef CAS PubMed.
- C. Sand, S. L. Peters, E. M. Pfaff and P. A. van-Zwieten, Effects of hypochlorite and hydrogen peroxide on cardiac autonomic receptors and vascular endothelial function, Clin. Exp. Pharmacol. Physiol., 2003, 30, 249–253 CrossRef CAS.
- V. Bauer, R. Sotnikova and D. Gergel, Comparison of the effects of activated neutrophils with the action of reactive oxygen species (ROS) on rat aortic smooth muscle (RASM), Prague Med. Rep., 2008, 109, S13–S14 Search PubMed.
- V. Jancinova, T. Perecko, R. Nosal, D. Mihalova, K. Bauerová and K. Drábiková, Pharmacological regulation of neutrophil activity and apoptosis. Contribution to new strategy for modulation of inflammatory processes, Interdiscip. Toxicol., 2011, 4, 11–14 CAS.
- B. M. Babior, Oxidants from phagocytes: agents of defense and destruction, Blood, 1984, 64, 959–966 CAS.
- Y. Nakamura, A. Murakami and H. Ohigashi, Search for naturally-occuring antioxidative chemo-preventers on the basis of the involvement of leukocyte-derived reactive oxygen species in carcinogenesis, Asian Pac. J. Cancer Prev., 2000, 1, 115–120 Search PubMed.
- A. Scalbert, I. T. Johnson and M. Saltmarsh, Polyphenols: antioxidants and beyond, Am. J. Clin. Nutr., 2005, 81, 215–217 Search PubMed.
- A. Gismondi, L. Canuti, S. Impei, G. Di Marco, M. Kenzo, V. Colizzi and A. Canini, Antioxidant extracts of African medicinal plants induce cell cycle arrest and differentiation in B16-F10 melanoma cells, Int. J. Oncol., 2013, 43, 956–964 Search PubMed.
- C. Forni, R. Braglia, N. Mulinacci, A. Urbani, M. Ronci, A. Gismondi, C. Tabolacci, B. Provenzano, A. Lentini and S. Beninati, Antineoplastic activity of strawberry (Fragaria × ananassa Duch.) crude extracts on B16-F10 melanoma cells, Mol. BioSyst., 2014, 10, 1255–1263 RSC.
- M. N. Rejeb, Le caroubier en Tunisie: situations et perspectives d'amélioration, Dans Quel avenir pour l'amélioration des plantes?, ed. AUPELF-UREF, John LibbeyEurotext, Paris, 1995, pp. 79–85 Search PubMed.
- L. A. Turnbull, L. Santamaria, T. Martorell, J. Rallo and A. Hector, Seed size variability: from carob to carats, Biol. Lett., 2006, 2, 397–400 CrossRef PubMed.
- I. Battle and J. Tous, Carob Tree (Ceratonia siliqua L.), International Plant Genetic Resources Institute, International Plant Genetic Resources Institute, Via delle Sette Chiese 142 00145, Rome, Italy, 1997 Search PubMed.
- H. Sebai, A. Souli, L. Chehimi, K. Rtibi, M. Amri, J. El-Benna and M. Sakly, In vitro and in vivo antioxidant properties of Tunisian carob (Ceratonia siliqua L.), J. Med. Food Res., 2013, 7, 85–90 CAS.
- B. Haber, Carob fibre benefits and applications, Cereal Foods World, 2002, 47, 365–369 CAS.
- K. Dornisch, S. Hippeli and B. H. Haber, Fiber preparation from carob with high antioxidative activity, dietary fibre in health and disease, Proceedings of the 7th International Vahouny Symposium, 2002, Abstract 11 Search PubMed.
- J. El Benna, L. P. Faust, J. L. Johnson and B. M. Babior, Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping: phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase, J. Biol. Chem., 1996, 271, 6374–6378 CrossRef CAS PubMed.
- T. Boussetta, M. A. Gougerot-Pocidalo, G. Hayem, S. Ciappelloni, H. Raad, R. Arabi-Derkawi, O. Bournier, Y. Kroviarski, X. Z. Zhou, J. S. Malter, P. K. Lu, A. Bartegi, P. M. Dang and J. El-Benna, The prolyl-isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils, Blood, 2010, 116, 5795–5802 CrossRef CAS PubMed.
- C. Proestos, I. S. Boziaris, G. J. E. Nycha and M. Komaitis, Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity, Food Chem., 2006, 95, 664–671 CrossRef CAS PubMed.
- A. M. Nuutila, K. Kammiovirta and K. M. Oksman-Caldentey, Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis, Food Chem., 2002, 76, 519–525 CrossRef CAS.
- J. El-Benna and P. M. Dang, Analysis of protein phosphorylation in human neutrophils, Methods Mol. Biol., 2007, 412, 85–96 CAS.
- P. M. Dang, A. Stensballe, T. Boussetta, H. Raad, C. Dewas, Y. Kroviarski, G. Hayem, O. N. Jensen, M. A. Gougerot-Pocidalo and J. El-Benna, A specific p47phox–serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites, J. Clin. Invest., 2006, 116, 2033–2043 CrossRef CAS PubMed.
- L. Udby and N. Borregaard, Subcellular fractionation of human neutrophils and analysis of subcellular markers, Methods Mol. Biol., 2007, 412, 35–56 CAS.
- L. F. Li, C. T. Yang, C. C. Huang and Y. Y. Liu, Low-molecular-weight heparin reduces hyperoxia augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B, Respir. Res., 2011, 12, 90 CrossRef CAS PubMed.
- A. Navas-Diaz, F. Garcia-Sanchez and J. A. Gonziilez-Garcia, Hydrogen peroxide assay by using enhanced chemiluminescence of the luminol H2O2–horseradish peroxidase system: Comparative studies, Anal. Chim. Acta, 1996, 327, 161–165 CrossRef.
- A. K. Papadakis and K. A. Roubelakis-Angelakis, The generation of active oxygen species differs in Nicotiana and Vitis plant protoplasts, Plant Physiol., 1999, 121, 197–205 CrossRef CAS PubMed.
- U. K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, 227, 680–685 CrossRef CAS PubMed.
- A. Umeno, M. Takashima, K. Murotomi, Y. Nakajima, T. Koike, T. Matsuo and Y. Yoshida, Radical-scavenging Activity and Antioxidative Effects of Olive Leaf Components Oleuropein and Hydroxytyrosol in Comparison with Homovanillic Alcohol, J. Oleo Sci., 2015, 64, 793–800 CrossRef CAS PubMed.
- F. Rodrigues, J. Santos, F. B. Pimentel, N. Braga, A. Palmeira-de-Oliveira and M. B. Oliveira, Promising new applications of Castanea sativa shell: nutritional composition, antioxidant activity, amino acids and vitamin E profile, Food Funct., 2015, 6, 2854–2860 CAS.
- D. R. Ambruso and R. B. Johnston, Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions and an enzymatic generating system, J. Clin. Invest., 1981, 67, 352–360 CrossRef CAS PubMed.
- P. Wang-Iverson, K. B. Pryzwanski, J. K. Spitznagel and M. H. Cooney, Bactericidal capacity of phorbolmyristate acetate-treated human polymorphonuclear leukocytes, Infect. Immun., 1978, 22, 945–955 CAS.
- L. Corsi, R. Avallone, F. Cosenza, F. Farina, C. Baraldi and M. Baraldi, Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line, Fitoterapia, 2002, 73, 674–684 CrossRef CAS.
- S. Kumazawa, M. Taniguchi, Y. Suzuki, M. Shimura, M. S. Kwon and T. Nakayama, Antioxidant activity of polyphenols in carob pods, J. Agric. Food Chem., 2002, 50, 373–377 CrossRef CAS PubMed.
- D. P. Makris and P. Kefalas, Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants, Food Technol. Biotechnol., 2004, 42, 105–108 CAS.
- M. Papagiannopoulos, H. R. Wollseifen, A. Mellenthin, B. Haber and R. Galensa, Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS, J. Agric. Food Chem., 2004, 52, 3784–3791 CrossRef CAS PubMed.
- M. A. Temiz, A. Temur and İ. Çelik, Antioxidant Role and Hepatoprotective Effects of Carob (Ceratonia siliqua L.) Seeds against Ethanol-Induced Oxidative Stress in Rats, J. Food Nutr. Res., 2015, 3, 57–61 Search PubMed.
- F. Chen, L. H. Qian, B. Deng, Z. M. Liu, Y. Zhao and Y. Y. Le, Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress, CNS Neurosci. Ther., 2013, 19, 675–681 CrossRef CAS PubMed.
- Y. Tang, J. Xu, W. Qu, X. Peng, P. Xin, X. Yang, C. Ying, X. Sun and L. Hao, Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms, J. Nutr. Biochem., 2012, 23, 1410–1416 CrossRef CAS PubMed.
- J. Pincemail, A. Thirion and M. Dupuis, Ginkgo biloba extracts inhibits oxygen species production generated by phorbolmyristate acetate stimulated human leukocytes, Experientia, 1987, 43, 181–184 CrossRef CAS.
- F. Y. Lin, Y. H. Chen, Y. L. Chen, T. C. Wu, C. Y. Li, J. W. Chen and S. J. Lin, Ginkgo biloba extract inhibits endotoxin-induced human aortic smooth muscle cell proliferation via suppression of toll-like receptor 4 expression and NADPH oxidase activation, J. Agric. Food Chem., 2007, 55, 1977–1984 CrossRef CAS PubMed.
- J. K. Lin, P. C. Chen, C. T. Ho, S. Lin and Y. Shoei, Inhibition of Xanthine Oxidase and NADPH Oxidase by Tea Polyphenol, J. Am. Chem. Soc., 2002, 18, 264–281 Search PubMed.
- E. Pastene, H. Speisky, M. Troncoso, J. Alarcón and G. Figueroa, In vitro inhibitory effect of apple peel extract on the growth of Helicobacter pylori and respiratory burst induced on human neutrophils, J. Agric. Food Chem., 2009, 57, 7743–7749 CrossRef CAS PubMed.
- D. A. Sawatzky, D. A. Willoughby, P. R. Colville-Nash and A. G. Ross, The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-x and Bax in the resolution of acute inflammation in vivo, Am. J. Pathol., 2006, 168, 33–41 CrossRef CAS PubMed.
- C. N. Serhan, S. D. Brain, C. D. Buckley, D. W. Gilroy, C. Haslett, L. A. O'Neill, M. Perretti, A. G. Rossi and J. L. Wallace, Resolution of inflammation: state of the art, definitions and terms, FASEB J., 2007, 21, 325–332 CrossRef CAS PubMed.
- J. M. Hallett, A. E. Leitch, N. A. Riley, R. Duffin, C. Haslett and A. G. Rossi, Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation, Trends Pharmacol. Sci., 2008, 29, 250–257 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |