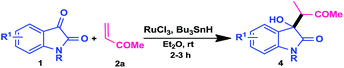A Ru(III) – catalyzed α-cross-coupling aldol type addition reaction of activated olefins with isatins†
A. Sanjeeva Kumara,
Palakuri Ramesha,
G. Santosh Kumara,
A. Swethaa,
Jagadeesh Babu Nanubolub and
H. M. Meshram*a
aMedicinal Chemistry and Pharmacology Division, Indian Institute of Chemical Technology, Hyderabad 500 007, India. E-mail: hmmeshram@yahoo.com; Fax: +91-40-27160512; Tel: +91-27191640
bLaboratory of X-ray Crystallography, Indian Institute of Chemical Technology, Hyderabad 500 007, India
First published on 14th December 2015
Abstract
A α-cross-coupling aldol type addition reaction activated olefins with isatins has been described in the presence of ruthenium(III) chloride and tributyltin hydride (TBTH) at room temperature. This method is found to work consistently for the delivering of ene-carbonyl coupled products in good to excellent yields with moderate to acceptable selectivity. The substrate scope of the present method was briefly discussed.
The reductive aldol reaction has received considerable attention in C–C bond formation because it does not require the pre-activation of nucleophiles and this process allows the coupling of two π-partners in one pot.1 Various catalysts have been used for reductive aldol coupling such as, rhodium,2 cobalt,3 iridium,4 ruthenium,5 palladium,6 copper7 and nickel.8 On the other hand, 3-substituted-3-hydroxy-2-oxindole entities are found in a large number of naturally occurring alkaloids with different biological activities such as potent anticancer, anti-HIV, antioxidant and neuroprotective properties.9 Selective representative examples are convolutamydines,10a donaxaridines,10b,c maremycins,10d dioxibras-sinines,10e celogentin K,10f 3-hydroxy hydroxyglucoisatisins,10g and TMC-95A10h (Fig. 1). Because of their structural significance in biological sciences, a number of strategies have been developed for the synthesis of such structural motifs, which includes allylation of isatins,11 nucleophilic addition to isatins,12 and direct hydroxylation of 3-substituted oxindoles.13 Though the synthesis of 3-substituted oxindoles is reported, the synthesis of alkyl 2-(3-hydroxy-2-oxoindolin-3-yl)propanoates or 3-hydroxy-3-(3-alkyl)indolin-2-ones or 2-(3-hydroxy-2-oxoindolin-3-yl)propanenitriles remain unexplored. So, it is desirable to develop efficient and general method using readily available catalyst.
However, to the best of our knowledge this is the first example of reductive coupling of activated olefins with isatins for the synthesis of alkyl 2-(3-hydroxy-2-oxoindolin-3-yl)propanoate, 3-hydroxy-3-(3-alkyl)indolin-2-one and 2-(3-hydroxy-2-oxoindolin-3-yl)propanenitrile derivatives.
Generally, the coupling reaction between activated olefins with an isatin in the presence of a base gives the Baylis–Hillman14 type products in which a C–C bond was formed between the α-carbon atom of the activated olefin and the C3-carbon atom of the indoline-2,3-dione. In our previous communication, we have endeavored β-cross coupling aldol type addition reaction of activated olefins with isatins/isatinimines in presence of In/Fe(III) catalytic system.15 Based on our continuing interest in the synthesis of 3-substituted-3-hydroxy-2-oxindole,16 herein we would like to disclose the α-cross-coupling aldol type addition reaction of range of activated olefins (methyl vinyl ketone, methyl/ethyl acrylates, acrylonitrile and cyclohexenone) with isatins to afford the valuable 3-funtionalized-3-hydroxy oxindoles in a single step by using RuCl3/TBTH catalytic system at room temperature (Scheme 1).
Results and discussions
To find out the reaction conditions, our initial investigations were focused on the reductive ene-carbonyl coupling of methyl vinyl ketone 2a (1.2 mmol) with an isatin 1 (1 mmol) using TBTH as hydrogen source (1.5 mmol) at room temperature and the results are summarized in Table 1. Initially, different metal catalysts were tested for this transformation and it was found that the RuCl3 is the most effective catalyst for driving the reaction to achieve the product in high yield as a mixture of diastereomers with 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 selectivity (Table 1, entry 6). The diastereomeric ratio of the resultant product was analyzed by the 1H NMR of the crude product. Whereas other tested metal catalysts were led to poor results (Table 1, entries 1–5 and 11–14) and no reaction was observed in the absence of metal catalyst (Table 1, entry 7). It indicates that the importance of the metal catalyst in the present method. With the RuCl3 as optimal catalyst, we next investigated the influence of the solvents. Among the different screened solvents, the best result was achieved when the reaction was performed in diethyl ether (Table 1, entry 6) and other solvents were showed poor results (Table 1, entries 9 and 10). To further optimize the reaction conditions, we were decided to investigate the effect of catalyst loading. Finally, the excellent result was obtained with 5 mol% of the catalyst (RuCl3) (Table 1, entry 6). When the catalyst (RuCl3) loading was decreased to 2 mol%, the product 4a was formed in lower yield (Table 1, entry 8). The structure of the expected reductive aldol product 4a was confirmed by spectroscopic data (1H, 13C, DEPT, IR and Mass) (Scheme 2).
5 selectivity (Table 1, entry 6). The diastereomeric ratio of the resultant product was analyzed by the 1H NMR of the crude product. Whereas other tested metal catalysts were led to poor results (Table 1, entries 1–5 and 11–14) and no reaction was observed in the absence of metal catalyst (Table 1, entry 7). It indicates that the importance of the metal catalyst in the present method. With the RuCl3 as optimal catalyst, we next investigated the influence of the solvents. Among the different screened solvents, the best result was achieved when the reaction was performed in diethyl ether (Table 1, entry 6) and other solvents were showed poor results (Table 1, entries 9 and 10). To further optimize the reaction conditions, we were decided to investigate the effect of catalyst loading. Finally, the excellent result was obtained with 5 mol% of the catalyst (RuCl3) (Table 1, entry 6). When the catalyst (RuCl3) loading was decreased to 2 mol%, the product 4a was formed in lower yield (Table 1, entry 8). The structure of the expected reductive aldol product 4a was confirmed by spectroscopic data (1H, 13C, DEPT, IR and Mass) (Scheme 2).
| Entry | Catalyst (mol%) | Solvent | Time | Yieldb (%) | dr (anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn)c syn)c |
|---|---|---|---|---|---|
| a Reaction conditions: isatin 1a (1 mmol), methyl vinyl ketone 2a (1.2 mmol), catalyst (X mol%) and TBTH (1.5 mmol) in 5 mL of solvent at room temperature.b Isolated yield.c Diastereomeric ratio. | |||||
| 1 | CuCl (5) | Et2O | 10 h | 65 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| 2 | Ni(acac)2 (5) | Et2O | 20 h | 35 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
| 3 | Cu(OAc)2·H2O (5) | Et2O | 20 h | 20 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 4 | FeCl3 (5) | Et2O | 30 h | 25 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
| 5 | InCl3 | Et2O | 24 h | 10 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 6 | RuCl3 (5) | Et2O | 3 h | 90 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 7 | — | Et2O | 24 h | No reaction | — |
| 8 | RuCl3 (2) | Et2O | 10 h | 45 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 9 | RuCl3 (5) | PhCH3 | 24 h | 30 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 10 | RuCl3 (5) | C6H6 | 10 h | 60 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 11 | CoC12 (2) | Et2O | 20 h | 40 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
| 12 | ZrCl4 (5) | Et2O | 24 h | 25 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
| 13 | Fe(acac)3 (5) | Et2O | 24 h | 30 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 14 | NiCl2 (5) | Et2O | 24 h | 40 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
By employing the optimal reaction conditions, we were keen to examine the generality of this reaction by treating a variety of isatins with enones (methyl vinyl ketone/cylohexenone 2a/2b) and the results are depicted in Tables 2&3. Initially, the ene-carbonyl coupling reaction of 2a and 2b with simple isatin 1 underwent smoothly and resulted into expected product as inseparable diastereomeric mixture with high yield (Table 3, 4a and 4l). Isatins bearing substituents with diverse electronic properties at the 5th position were successfully coupled with 2a and 2b in the present protocol. For example, the reaction of 5-halo isatins proceeded smoothly and afforded corresponding products in high yields with the variable selectivity (Tables 2&3, 4b–4e and 4m–4p). Other 5-substitued isatins were also showed fine reactivities and furnished the aldol products in high yields with poor to good selectivity (Tables 2&3, 4f, 4g and 4q, 4r). In addition to this, 4-substituted, 4,6-disubstituted, 4,7-disubstituted and 5,7-disubstituted isatins were also participated in this reaction with 2a/2b and gave comparatively with moderate yields of the products (Tables 2&3, 4i–4k and 4s–4u). Furthermore, N-protected isatins were also successfully cross-coupled with 2a/2b to offer the corresponding products in acceptable yields (Tables 2&3, 4h and 4v–4x).
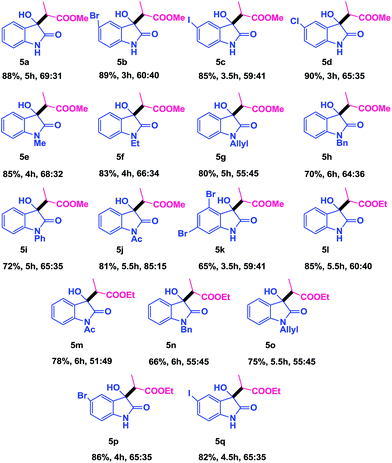
The scope of this method was further supported by examining a methyl/ethyl acrylates 3a/3b in place of enones and the results are presented in Table 4. At first, the simple isatin underwent the reductive coupling reaction with 3a/3b and provided an ene-carbonyl coupled product in high yield with poor selectivity (Table 4, 5a and 5l). It was found that a range of isatins were reacted in an efficient manner with 3a/3b under the optimized reaction conditions. For example, the 5-halo isatins were took place smoothly and afforded the corresponding products in high yields nearly with the same selectivity (Table 4, 5b–5d and 5p, 5q). In addition to simple and 5-substituted isatins, 4,6-disubstituted and various N-substituted isatins were also coupled with 3a/3b to produce the cross-coupled products in moderate to good yields (Table 4, 5e–5k and 5m–5o).
Inspired by the above achievement from the reductive ene-carbonyl coupling of enones and acrylates with isatin, we then turned our attention to apply this method to acrylonitrile 3c and the results are depicted in Table 5. As shown in Table 5, it was found that a variety of isatins were reacted well with coupling partner 3c under the standard reaction conditions. For example, simple isatin smoothly participated in the reductive ene-carbonyl coupling reaction and gave the coupled product in good yield with moderate selectivity (Table 5, 5r). Other isatins like 5-F, 5-Br and 5-I isatins also furnished the aldol adducts in acceptable yields (Table 5, 5s–5u).
We have proposed the reaction mechanism for reductive ene-carbonyl coupling reaction based on previous reports17 (Scheme 3). Initially, TBTH reacts with RuCl3 and gave the ruthenium hydride complex a. The 1,4-addition of ruthenium hydride species to methyl vinyl ketone 2a gives the ruthenium enolate b. Next, the aldol addition reaction of ruthenium enolate to an isatin offers a (2-oxo-3-(3-oxobutan-2-yl)indolin-3yloxy) ruthenium(III) chloride c and is followed by transmetallation with TBTH, results in the formation of a 3-(3-oxobutan-2-yl)-3-(tributylstannyloxy)indolin-2-one d. Finally the quenching of the d with aq. NH4Cl produces reductive coupling product 4a. We have observed that the generation of metal-hydride species is essential for this method.
The structure of one of the products 4r was further confirmed by a single-crystal X-ray diffraction analysis (Fig. 2). Based on the single-crystal structure we have identified major isomer as anti.
Conclusion
In summary, we have established the ruthenium(III) catalyzed an efficient α-cross-coupling aldol type addition reaction of activated olefins with isatins by using TBTH at room temperature. Our strategy has proven to be quite general, atom-economical and provides easy access for the synthesis of a wide variety of 3-functionalized-3-hydroxy oxindoles. Biological evaluations of our synthetic molecules are currently under progress in our lab.Acknowledgements
A. S. K., P. R., G. S. K., and A. S. thank the CSIR-UGC India for award of a fellowship and Dr A. Kamal, Outstanding scientist and Head of MCP Division, for his support and encouragement. The authors thank CSIR-India for financial support as part of XII five year plan programme under title ORIGIN CSC 0108.References
- (a) H.-Y. Jang, R. R. Huddleston and M. J. Krische, Chemtracts, 2003, 16, 554 CAS; (b) H.-Y. Jang and M. J. Krische, Eur. J. Org. Chem., 2004, 19, 3953 CrossRef; (c) H.-Y. Jang and M. J. Krische, Acc. Chem. Res., 2004, 37, 653 CrossRef CAS PubMed; (d) P. Chiu, Synthesis, 2004, 2210 CrossRef CAS; (e) M.-Y. Ngai, J.-R. Kong and M. J. Krische, J. Org. Chem., 2007, 72, 1063 CrossRef CAS PubMed.
- (a) A. Revis and T. K. Hilty, Tetrahedron Lett., 1987, 28, 4809 CrossRef CAS; (b) I. Matsuda, K. Takahashi and S. Sato, Tetrahedron Lett., 1990, 31, 5331 CrossRef CAS; (c) S. J. Taylor and J. P. Morken, J. Am. Chem. Soc., 1999, 121, 12202 CrossRef CAS; (d) C.-X. Zhao, J. Bass and J. P. Morken, Org. Lett., 2001, 3, 2839 CrossRef CAS PubMed; (e) S. J. Taylor, M. O. Duffey and J. P. Morken, J. Am. Chem. Soc., 2000, 122, 4528 CrossRef CAS.
- (a) T. G. Baik, A. L. Luis, L. C. Wang and M. J. Krische, J. Am. Chem. Soc., 2001, 123, 5112 CrossRef CAS PubMed; (b) L. C. Wang, H.-Y. Jang, Y. Roh, V. Lynch, A. J. Schultz, X. Wang and M. J. Krische, J. Am. Chem. Soc., 2002, 124, 9448 CrossRef CAS PubMed; (c) H. W. Lam, P. M. Joensuu, G. J. Murray, E. A. F. Fordyce, O. Prieto and T. Luebbers, Org. Lett., 2006, 8, 3729 CrossRef CAS PubMed.
- C. X. Zhao, M. O. Duffey, S. J. Taylor and J. P. Morken, Org. Lett., 2001, 3, 1829 CrossRef CAS PubMed.
- T. Doi, T. Fukuyama, S. Minamino and I. Ryu, Synlett, 2006, 18, 3013 Search PubMed.
- S. I. Kiyooka, A. Shimizu and S. Torii, Tetrahedron Lett., 1998, 39, 5237 CrossRef CAS.
- (a) P. Chiu, B. Chen and K. F. Cheng, Tetrahedron Lett., 1998, 39, 9229 CrossRef CAS; (b) D. Zhao, K. Oisaki, M. Kanai and M. Shibasaki, Tetrahedron Lett., 2006, 47, 1403 CrossRef CAS; (c) O. Chuzel, J. Deschamp, C. Chausteur and O. Riant, Org. Lett., 2006, 26, 5943 CrossRef PubMed.
- C. C. Chrovian and J. Montgomery, Org. Lett., 2007, 3, 537 CrossRef PubMed.
- For reviews of oxindole alkaloids, see: (a) S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., 2007, 119, 8902 CrossRef; (c) K. Monde, K. Sasaki, A. Shirata and M. Tagusuki, Phytochemistry, 1991, 30, 2915 CrossRef CAS; (d) M. Suchy, P. Kutschy, K. Monde, H. Goto, N. Harada, M. Takasugi, M. Dzurilla and M. Balentova, J. Org. Chem., 2001, 66, 3940 CrossRef CAS PubMed; (e) T. Bui, S. Syed and C. F. Barbas, J. Am. Chem. Soc., 2009, 131, 8758 CrossRef CAS PubMed.
- (a) Y. Kamano, H. P. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Tetrahedron Lett., 1995, 36, 2783 CrossRef CAS; (b) H. B. Rasmussen and J. K. MacLeod, J. Nat. Prod., 1997, 60, 1152 CrossRef CAS; (c) T. Kawasaki, M. Nagaoka, T. Satoh, A. Okamoto, R. Ukon and A. Ogawa, Tetrahedron, 2004, 60, 3493 CrossRef CAS; (d) W. Balk-Bindseil, E. Helmke, H. Weyland and H. Laatsch, Liebigs Ann., 1995, 1291 CrossRef CAS; (e) K. Monde, K. Sasaki, A. Shirata and M. Tagusuki, Phytochemistry, 1991, 30, 2915 CrossRef CAS; (f) H. Suzuki, H. Morita, M. Shiro and J. Kobayashi, Tetrahedron, 2004, 60, 2489 CrossRef CAS; (g) A. Frechard, N. Fabre, C. Pean, S. Montaut, M. T. Fauvel, P. Rollin and I. Fouraste, Tetrahedron Lett., 2001, 42, 9015 CrossRef CAS; (h) J. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Kuroda, R. Shimizu, T. Ohnuki and S. Komatsubara, J. Org. Chem., 2000, 65, 990 CrossRef CAS PubMed.
- (a) X.-C. Qiao, S.-F. Zhu and Q.-L. Zho, Tetrahedron: Asymmetry, 2009, 20, 1254 CrossRef CAS; (b) T. M. Jared, V. H. Nadine, E. M. Maximillian, P. C. Stephen and T. S. Jared, Org. Lett., 2013, 15, 5615 CrossRef PubMed; (c) Z.-Y. Cao, Y. Zhang, C.-B. Ji and J. Zhou, Org. Lett., 2011, 13, 6398 CrossRef CAS PubMed; (d) A. Benito, A. Pedro and R.-A. Raquel, J. Org. Chem., 2005, 70, 3198 CrossRef PubMed.
- (a) V. H. Nadine, C. T. Yng, T. T. Ngon and K. F. Annaliese, Org. Lett., 2012, 14, 2218 CrossRef PubMed; (b) Z. Y. Cao, Y. Zhang, C. B. Ji and J. Zhou, Org. Lett., 2011, 13, 6398 CrossRef CAS PubMed; (c) X. C. Qiao, S. F. Zhu and Q. L. Zhou, Tetrahedron: Asymmetry, 2009, 20, 1254 CrossRef CAS; (d) N. V. Hanhan, A. H. Sahin, T. W. Chang, J. C. Fettinger and A. K. Franz, Angew. Chem., Int. Ed., 2010, 49, 744 CrossRef CAS PubMed; (e) J. Deng, S. Zhang, P. Ding, H. Jiang, W. Wang and J. Li, Adv. Synth. Catal., 2010, 352, 833 CrossRef CAS.
- (a) D. Sano, K. Nagata and T. Itoh, Org. Lett., 2008, 10, 1593 CrossRef CAS PubMed; (b) T. Bui, N. R. Candeias and C. F. Barbas III, J. Am. Chem. Soc., 2010, 132, 5574 CrossRef CAS PubMed; (c) T. Ishimaru, N. Shibata, J. Nagai, S. Nakamura, T. Toru and S. Kanemasa, J. Am. Chem. Soc., 2006, 128, 16488 CrossRef CAS PubMed.
- (a) F. Zhong, G.-Y. Chen and Y. Lu, Org. Lett., 2011, 13, 82 CrossRef CAS PubMed; (b) Y.-L. Liu, B.-L. Wang, J.-J. Cao, L. Chen, Y.-X. Zhang, C. Wang and J. Zhou, J. Am. Chem. Soc., 2010, 132, 15176 CrossRef CAS PubMed; (c) Y. M. Chung, Y. J. Im and J. N. Kim, Bull. Korean Chem. Soc., 2002, 23, 1651 CrossRef CAS; (d) S. C. Kim, S. Gowrisankar and J. N. Kim, Tetrahedron Lett., 2006, 47, 3463 CrossRef CAS; (e) S. C. Kim, K. Y. Lee, S. Gowrisankar and J. N. Kim, Bull. Korean Chem. Soc., 2006, 27, 1133 CrossRef CAS.
- A. S. Kumar, P. Ramesh, G. S. Kumar, J. B. Nanubolu, T. P. Rao and H. M. Meshram, RSC Adv., 2015, 5, 51581 RSC.
- (a) H. M. Meshram, D. A. Kumar, P. R. Goud and B. C. Reddy, Synth. Commun., 2010, 40, 39 CrossRef CAS; (b) H. M. Meshram, P. Ramesh, B. C. Reddy, B. Sridhar and J. S. Yadav, Tetrahedron, 2011, 67, 3150 CrossRef CAS; (c) H. M. Meshram, P. Ramesh, B. C. Reddy and G. S. Kumar, Chem. Lett., 2011, 4, 357 CrossRef; (d) H. M. Meshram, P. Ramesh, A. S. Kumar and A. Swetha, Tetrahedron Lett., 2011, 52, 5862 CrossRef CAS; (e) H. M. Meshram, N. N. Rao, L. C. Rao and N. S. Kumar, Tetrahedron Lett., 2012, 53, 3963 CrossRef CAS; (f) B. T. Pramod, K. Sirisha, A. V. S. Sarma, J. N. Babu and H. M. Meshram, Tetrahedron, 2013, 69, 6415 CrossRef; (g) B. T. Pramod and H. M. Meshram, RSC Adv., 2014, 4, 6019 RSC; (h) B. T. Pramod and H. M. Meshram, RSC Adv., 2014, 4, 5343 RSC.
- (a) E. Yamaguchi, J. Mowat, T. Luong and M. J. Krische, Angew. Chem., Int. Ed., 2013, 52, 8428 CrossRef CAS PubMed; (b) D. Zhao, K. Oisaki, M. Kanai and M. Shibasaki, Tetrahedron Lett., 2006, 47, 1403 CrossRef CAS; (c) J. Joseph, F. Jaroschik, D. Harakat, K. V. Radhakrishnan, J. Vasse and J. Szymoniak, Chem.–Eur. J., 2014, 20, 5433 CrossRef CAS PubMed; (d) K. Sato, M. Isoda, Y. Tokura, K. Omura, A. Tarui, M. Omote, I. Kumadaki and A. Ando, Tetrahedron Lett., 2013, 54, 5913 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1435101. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14714j |
| This journal is © The Royal Society of Chemistry 2016 |