Enhanced catalytic ability of chitosan–Cu–Fe bimetal complex for the removal of dyes in aqueous solution†
Abstract
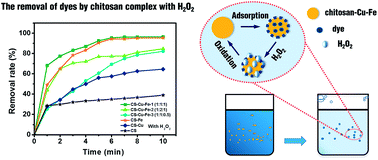
In this study, despite the high adsorption ability, efficient catalytic activity of a chitosan–metal complex has been developed through the chelation of chitosan polymer with bimetals Cu(II) and Fe(III). The removal of C. I. Reactive Black 5 (RB 5) by the chitosan–Cu–Fe complex/H2O2 system was studied in the pH range from 4 to 12. The maximal dye removal rate was achieved at an optimal concentration of Cu and Fe in the chitosan–Cu–Fe matrix, demonstrating the combination of sorptive enrichment and catalytic degradation. The results indicated that TOC removal and discoloration of the dye achieved 89.9% and 96.5% in a short reaction time. The pH sensitivity of the chitosan complex, the effect of the coexisting ions and the adsorption of other anionic dyes were also studied. X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) were analyzed to study the structure of the chitosan bi-metal complex and a possible mechanism was proposed.

 Please wait while we load your content...
Please wait while we load your content...