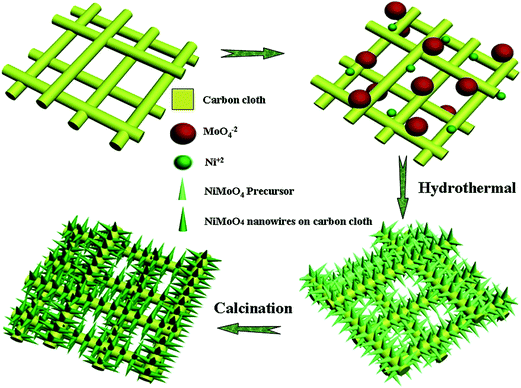
β-NiMoO4 nanowire arrays grown on carbon cloth for 3D solid asymmetry supercapacitors†
Chuanshen Wang,
Yi Xi*,
Chenguo Hu,
Shuge Dai,
Mingjun Wang,
Lu Cheng,
Weina Xu,
Guo Wang and
Wenlong Li
Department of Applied Physics, Chongqing University, Chongqing, 400044, China. E-mail: yxi6@cqu.edu.cn; Fax: +86 23 65678362; Tel: +86 23 65678362
First published on 26th November 2015
Abstract
Based on β-NiMoO4 nanowire (NW) arrays grown on carbon cloth as the electrode materials, a 3D solid asymmetrical supercapacitor has been fabricated. The produced β-NiMoO4 NW arrays on carbon cloth compared to the power of NiMoO4 nanowires deposited directively on carbon cloth can increase the efficiency of nanomaterials participating in reactions. The cone-shaped NW arrays have a high specific surface area (99 m2 g−1), which can provide more electroactive sites for Li+ and enhance conductivity through providing short transport and diffusion paths for both ions and electrons. And the cylindrical supercapacitor can allow more β-NiMoO4 NW arrays to saturate with electrolyte to enhance the properties of the supercapacitor. Furthermore, the electrode has a highest energy density of 36.86 W h kg−1, a maximum power density of 1100 W kg−1, and a large capacitance of 414.7 F g−1 at a current density of 0.25 A g−1, all of which demonstrate excellent behavior. And the capacitance of the supercapacitor reached 65.96% of the initial capacitance over 6000 cycles. All of these results indicated that the β-NiMoO4 NW arrays grown on carbon cloth could be a promising candidate for high performance supercapacitors.
1. Introduction
With the proliferation of electronic devices, the requirement for improving the properties of portable power supplies is becoming increasingly evident. Energy storage devices such as batteries and supercapacitors are an indispensable part of our current society.1,2 Supercapacitors, also named as electrochemical capacitors (ECs) or ultra-capacitors, afford very promising potential for energy storage applications due to their advantages, which include long cycle life, high power density, long standing time, environmental friendliness, safety and so on. Supercapacitors can be classified into two varieties, according to their storage mechanisms,3,4 electrical double layer capacitors (EDLCs) and pseudo-capacitors.5,6 These two mechanisms can function simultaneously, relying on the nature of the electrode materials. In general, carbon materials, because of their high specific surface area, are applied in EDLCs. Conducting polymers and metal oxides are used for pseudocapacitors.7–9 To obtain high power density and cycling stability, appropriate nanomaterials are essential.10–12 Moreover, supercapacitors have suitable properties and flexibility which can meet various design and power needs of modern gadgets.13,14 At the same time, according to the energy density (E) of supercapacitors, its equation can be given as follows: E = 1/2CV2. The way of enhancing its capacitance is by developing novel electrode materials or expanding the potential window.15,16 Due to metal oxide’s special capacitive properties of higher capacitance than most conductive polymers, considerable research efforts have been focused on developing metal oxide materials. Metal oxide materials can be divided into three categories: (a) single metal oxides such as NiO, Co3O4, MnO2, RuO2, etc.,17–20 (b) binary metal oxides, such as NiCo3O4, NiMoO4, CoMoO4, etc.,21–23 and (c) hybrid nanostructures, such as Co3O4@NiMoO4, NiCo3O4@MnO2, MnO2/KCu7S4, etc.24–26 Crystalline nickel molybdate (NiMoO4) has three polymorphic forms, and two of them, α and β, are stable under standard pressure conditions. α-NiMoO4 and β-NiMoO4 are both monoclinic but have different Mo coordination modes, that is to say, they have octahedral and tetrahedral sites, respectively.27–29 Nanocrystalline NiMoO4 has lots of advantages, such as short diffusion path lengths, pseudocapacitive contributions to charge storage, and the stability of the materials to more easily accommodate the strain from lithium intercalation.28 Furthermore, based on NiMoO4 nanowire (NW) arrays grown on carbon cloth, it has good electrical conductivity, low-cost, flexibility and large specific surface area. So, materials based on NiMoO4 NW arrays grown on carbon cloth are candidate electrode materials due to their good electrochemical properties.29In our work, based on NiMoO4 NW arrays grown on carbon cloth, we report that the high performance 3D all-solid-state cylindrical supercapacitor is different from the reported liquid supercapacitor.30 The β-NiMoO4 NW arrays which were grown on a carbon cloth electrode display a wide potential window, high power density and good cycling stability for supercapacitors. This kind of supercapacitor is an asymmetrical cylinder, which consists of a cathode of β-NiMoO4 NW arrays grown on carbon cloth and an anode of β-NiMoO4 NW arrays grown on carbon wires twisted onto a carbon rod. Furthermore, the supercapacitor can power one light-emitting diode (LED) for about 260 seconds. This type of supercapacitor is a solid-supercapacitor, which will overcome the major disadvantages of conventional liquid supercapacitors. It is pivotal for device integration and environmental friendliness. All this research indicates that the β-NiMoO4 NW arrays are ideal materials for supercapacitor electrode materials, and the all-solid-state asymmetrical cylinder supercapacitor has excellent capacitance performance.
2. Experimental section
2.1 Synthesis of β-NiMoO4 NW arrays on carbon cloth
The approach of β-NiMO4 NW array synthesis on carbon cloth is a facile hydrothermal method, which has minor differences from the literature.31 The reagents were used without further purification in the experiments and were all of analytical grade. Prior to the synthesis, the carbon cloth substrate (length × width = 2 cm × 3 cm) and carbon fiber were rinsed with ammonia water (pH = 7) and water, respectively. The washed carbon cloth and carbon fiber were dried in an oven for 30 minutes and were immersed in 5% KMnO4 solution for 40 minutes. Then they were placed in a Teflon-lined autoclave. 2.5 mmol of Ni (NO3)2·6H2O and 2.5 mmol of Na2MoO4·2H2O were mixed in 50 ml of ultra-pure water under constant magnetic stirring until the mixture solution became clear. The mixture solution was transferred into Teflon-lined stainless steel autoclave liners and the treated carbon cloth and carbon fiber were immersed in the mixture solution. The mixture solution was divided into three parts whose volumes all are 17 mL. They all were sealed into Teflon-lined stainless steel autoclave liners and maintained at 100 °C, 140 °C and 180 °C for 8 h. After cooling down to room temperature, the carbon cloth, which had a very light green precipitate, was taken out and washed with distilled water and alcohol by ultra-sonication. When all of the residual nanoparticles were removed, the carbon cloth was dried at 55 °C for 5 h and annealed at 450 °C for 2 h in air. The above procedures were repeated and the temperature was set at 140 °C for 5, 8 and 11 h.2.2 Assembly of the solid-state supercapacitor
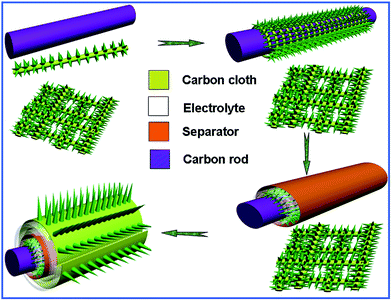
The β-NiMoO4 NW array 3D supercapacitor consisted of β-NiMoO4 NW arrays grown on carbon cloth, a solid electrolyte (polyvinyl alcohol PVA–LiCl gel), a separator and β-NiMoO4 NW arrays grown on carbon fibers twisted onto a carbon rod (β-NiMoO4 NWs on carbon cloth as the positive electrode and carbon thread as the negative electrode). The PVA–LiCl gel electrolyte was reported elsewhere.2 In the fabrication process, firstly one carbon thread was textile-wrapped around a carbon rod as the negative electrode of about 2 cm in length. Secondly, the electrode was coated with a layer of electrolyte (LiCl–PVA gel), and then was wrapped with a separator (Whatman 8 μm filter paper) which was coated with the electrolyte on both sides. Finally, one piece of carbon cloth (2 cm × 3 cm) was put uniformly on the electrolyte and was twinned with the previous processed electrode for the micro-supercapacitor.2.3 Characterization and electrochemical measurement
The surface microstructure and morphology of the sample were characterized using a scanning electron microscope (Nova 400 nano SEM) and a transmission electron microscope (TEM, TECNAI20 Philips). The specific surface area was measured using the multipoint Brunauer–Emmett–Teller (BET) method at 77.3 K with a Quantachrome NOVA4200e system. The pore size distribution was also obtained from the desorption isotherms using the Barrett–Joyner–Halenda (BJH) method. Energy-dispersive X-ray (EDS) spectroscopic analysis was performed using INCA, Oxford Instruments, combined with SEM. The carbon cloth X-ray diffraction (XRD) patterns were recorded on a BDX 3200 China with CuKα radiation. Cyclic voltammetry (CV) and galvanic charge–discharge (GCD) measurements were conducted using a chemical workstation (CHI760D). The electrochemical impedance spectroscopy (EIS) was carried out in a frequency range of 1–100 kHz at an open circuit potential of 0.01 V. Specific capacitances were calculated from the CV and charge–discharge curves by the way of eqn (1) and eqn (2), respectively. The area capacitance is calculated the same as eqn (1) and eqn (2), besides m being substituted by S. The energy density (E) and power density (P) are calculated according to eqn (3) and eqn (4).28–34
 | (1) |
 | (2) |
 | (3) |
 | (4) |
3. Results and discussion
3.1 Material characterization
In this study, a facile hydrothermal approach was used for the synthesis of β-NiMoO4 NW arrays grown on carbon cloth. The details are shown in the experimental part. The schematic illustration of the formation processes of the β-NiMoO4 NW arrays on carbon cloth is shown in Fig. 1. The chemical reaction equations are listed as follows.35–37
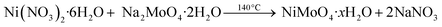 | (5) |
6
The crystal phase structures and purity of the β-NiMoO4 NWs on carbon cloth were investigated using X-ray diffraction. Fig. 2(a) shows XRD patterns of β-NiMoO4 supported on carbon cloth at different times at the same temperature (140 °C at 5, 8 and 11 h). The strong peak of XRD patterns is the typical peak of β-NiMoO4 products, however, which can not be distinguished from the main peak of the carbon cloth. But some inferior peaks ((02−1), (201), (11−2), (31−1), (112) and (22−1)), which are in good accordance with the monoclinic structure of β-NiMoO4 (JCPDS-no. 45-0142 with space group C2/m (12) and cell parameters a = 10.18 Å, b = 9.241 Å, c = 7.018 Å), can accurately confirm this kind of material.38 Moreover, the inset of Fig. 2(a) suggests that the as-prepared β-NiMoO4 has poor crystallinity and the existence of characteristic (11−2) is the typical characteristic peak of β-NiMoO4. The chemical components of β-NiMoO4 were characterized by EDS, as shown in Fig. 2(b). The elements are Ni, Mo, O and C, and the C is indexed to the carbon cloth substrate. The EDS pattern of this material has hardly any noise peaks, which indicates little electric charge effect. That is to say, the conductivity of β-NiMoO4 is good. To identify the existence of β-NiMoO4, TEM was carried out. From Fig. 2(c), the diameter of the β-NiMoO4 arrays was about 27–40 nm. Importantly, the high-resolution TEM image in Fig. 2(d) shows that the interplanar spacing is 0.31 nm and 0.35 nm, corresponding to the (220) and (310) planes of NiMoO4 in the standard PDF number (JCPDS-no. 45-0142). The different time (5, 8 and 11 h) SEM images of the β-NiMoO4 arrays at 140 °C are displayed in Fig. 3(a)–(c). The SEM images of the β-NiMoO4 arrays on carbon cloth at different temperatures (100, 140 and 180 °C) are shown in Fig. S1(a),† and 3(b) and (d). Compared with naked carbon cloth (Fig. S1(b)†), the rod-like structure of the β-NiMoO4 NW arrays was grown on the carbon fibers and the sample in Fig. 3(b) had a uniform structure and more spaces between the arrays than those in Fig. 3(a) and (d). From these results, we can see that the best growth condition is the growth time of 8 h at 140 °C. Meanwhile, nitrogen adsorption–desorption revealed that the specific surface area of the sample in Fig. 3(b) is 99 m2 g−1, which is higher than 18.438 m2 g−1 (Fig. 3(a)) and 25.361 m2 g−1 (Fig. 3(c)). The highest specific surface area of the sample in Fig. 3(b) is higher than many of those in the literature.39,40 And the pore diameter is about 3.73 nm from the BJH analysis.
 | ||
| Fig. 3 SEM images of the prepared β-NiMoO4 NW arrays on carbon cloth: (a), (b), and (c) at different hours at 140 °C (5, 8, and 11 h), and (d) SEM image of the β-NiMoO4 NW arrays at 180 °C. | ||
3.2 Electrochemical properties
To further demonstrate the excellent behavior of this β-NiMoO4 NW array electrode and investigate its potential application in a supercapacitor, CV curves, GCD curves, cycling performance and EIS spectra were obtained using a two-electrode solid electro-chemical cell containing the LiCl–PVA gel electrolyte. First, the electrode was fabricated, and the details are shown in the experimental part. Moreover, the loading mass of positive and negative materials is about 0.6 mg cm−2, 0.23 mg at 140 °C for 5 h, 0.62 mg cm−2, 0.25 mg at 140 °C for 8 h and 0.63 mg cm−2, 0.26 mg at 140 °C for 11 h. The fabrication process of the 3D supercapacitor is shown in Fig. 4. CV curves of this electrode with the potential ranging from 0 to 0.8 V were performed at different scan rates, as shown in Fig. 5. Each CV curve is composed of the quasi-rectangular profile, which indicates the ideal pseudo-capacitive properties of the electrode and a fast redox reaction with PVA/LiCl under a wide range of scan rates.38 Furthermore, the highest current response, up to −0.035 A to 0.035 A in the CV curves, displays the excellent charge storage ability and quick ion mobility between the electrode and electrolyte. As shown in Fig. 5(d), the EC at the synthesis time of 8 h has the highest performance, for having the biggest specific area. The β-NiMoO4 NW arrays grown on carbon cloth (2 cm × 2 cm) electrode, Pt foil and a standard calomel electrode were used as working electrode, counter electrode and reference electrode, respectively, by using a three-electrode measurement. Fig. S2(a–c)† show CV curves of β-NiMoO4 at different scan rates with the same time. Fig. S2(d)† shows the CV curves of β-NiMoO4 at a scan rate of 200 mV s−1 of different as-prepared NiMoO4 growth times. Each CV curve of β-NiMoO4 has strong redox peaks, indicating the pseudocapacitive characteristics of the as-prepared NiMoO4 electrode material.25,31 Lastly, the asymmetric redox peaks suggested that the β-NiMoO4 NW array electrode materials are unstable.GCD curves of NiMoO4 NW arrays supported on carbon cloth ECs (140 °C at 5, 8 and 11 h) with different discharge current densities ranging from 0.25 A g−1 to 2.75 A g−1 are shown in Fig. S3(a–c).† Fig. 6(a) shows the discharge time at a current density of 0.5 A g−1 (140 °C at 8 h) is the longest, which indicates the best performance. Fig. 6(b) shows the nearly triangular shape of charge–discharge cycles, showing high symmetry and long linear slope time at high discharge current values, which demonstrate the excellent conductivity and good capacitance. Presented in the inset of Fig. 6(b), it is observed that the discharge time can reach 42.5 seconds even at a current of 2.75 A g−1. On the basis of eqn (2), the specific capacitances of the ECs were calculated to be 414.7, 306.75, 247.03, 204.75, 170.16, and 139.22 F g−1 at current densities of 0.25 A g−1, 0.50 A g−1, 1.25 g A−1, 1.75 A g−1, 2.25 A g−1 and 2.75 A g−1, respectively, as shown in Fig. 6(c). Although the specific capacitance is much lower than the previous reports of NiMoO4 at the same scan rate, the reactions occur in a solid electrolyte.25,31,42 Moreover, the specific capacitance of β-NiMoO4 NW arrays on carbon cloth is very high compared to the specific capacitance of some metal oxide materials.43–45 The wide range of the scan rates shows that the Li+ of the electrolyte has enough time to diffuse between the electrolyte and the electrode. From the inset of Fig. 3(b), the products have both robust adhesion and electrical contact to the carbon fibers and a net structure which has lots of interspaces, matching with the high specific surface area.31 The spaces between the NWs are so many that they can make the electrolyte fully accessible. So the main reasons might be due to the higher porous structure of β-NiMoO4 NW arrays for easy diffusion of the electrolyte into the inner region of the electrodes and the high conductivity of the carbon cloth with multi-channels.41 Furthermore, the potential drop (IR drop) of about 0.03 V at the current density of 0.25 A g−1 at the beginning of the discharge cycle indicates a fast I–V response and low internal resistance (Rs) of the capacitor. The IR drop can account for the Rs of the products or the contact resistance between the electrode and electrolyte. Based on eqn (3) and eqn (4), the power density (P) and energy density (E) can be further calculated from the GCD curves of Fig. 6(b). For the β-NiMoO4 NW supercapacitor, the energy densities were 36.86, 27.27, 21.96, 18.2, 15.13 and 12.3 W h kg−1, and the power densities were 100, 300, 500, 700, 900 and 1100 W kg−1, respectively, which are displayed in Fig. 6(d).
Due to the NiMoO4 NW arrays being supported on carbon cloth, the electrochemical performances of the original carbon cloth were tested. Fig. S1(c) and (d)† demonstrate CV and GCD curves of the original carbon cloth. And the CV and GCD curves of NiMoO4 NW arrays supported on carbon cloth are displayed in Fig. 5(b) and 6(b). Compared to the CV and GCD curves of the original carbon cloth electrode, the β-NiMoO4 NW array electrode is far better. The reason is that the special structure of the supercapacitor and lots of mesopores in β-NiMoO4 guarantee that sufficient β-NiMoO4 NW arrays supported on carbon cloth participate in enhancing the capacitance of the supercapacitor. It is essential for practical, flexible, lightweight, portable and environmentally friendly devices. The 3D supercapacitor with β-NiMoO4 NW arrays is flexible and can be wound in a two-dimensional plane. Furthermore, it is assumed that if the carbon rod is replaced by a bronze rod, the supercapacitor can be curved at any angle without destroying its physical structure.
It is important to investigate the long term cycling stability of this kind of supercapacitor. The stability of supercapacitors has been studied by repeating the charge–discharge process at a potential ranging from 0 to 0.8 V. From Fig. 7, the coulombic efficiency is nearly 100% for every charge–discharge cycle. The value of specific capacitance is calculated with respect to the number of charge–discharge cycles at a constant 6 A g−1. The capacitance of the first 800 cycles decreased by about 5.3% and it increased at the beginning of the 800th until the end of the 2000th. The capacitance decreased with the cycle numbers and had 65.9% retention of the initial specific capacitance after 6000 cycles. The bad stability indicated that this material is not suitable enough during the long testing time. This is due to the diffusion of ions between the electrolyte and the active material of the electrode does not perform very well at high current density.
 | ||
| Fig. 7 Cycling stability of β-NiMoO4 NW arrays supported on carbon cloth at a current density of 6 A g−1 and the coulombic efficiency over 6000 cycles. | ||
Fig. 8 displays the EIS spectra in the frequency range of 1–100 kHz with an open circuit potential of 0.01 V. The measured EIS spectrum was analyzed using a Nyquist plot and ZSim software simulation method based on the equivalent circuit, which is also displayed in the inset of Fig. 8(a). The inset of Fig. 8(a) shows that the enlarged semicircle in a high frequency fitted well with the equivalent circle model. In the low frequency range, the line is not vertical. This indicates that the diffusion of ions between the electrolyte and the active material of the electrode is not very good. This property is in accordance with the cycling stability. The intersecting point with the real axis in the high frequency region shows that the Rs of the products includes ionic resistance, inherent resistance of the β-NiMoO4 NW arrays and the carbon cloth and contact resistance of the electrode materials and the electrolyte.1 The Rs of the β-NiMoO4 NW arrays grown on the carbon cloth is about 3.4 Ω, as shown in the inset of Fig. 8(a). The small semicircle indicates the fine electrical conductivity and small resistance of the material. The behavior of the EIS spectra is in accord with the small IR of the GCD curves. The charge transfer resistance (Rf) between the electrode and the electrolyte is about 1.4 Ω.
For the purpose of investigating the electrochemical stability, EIS was performed after finishing 6000 cycles, as presented in Fig. 8(b). The Rs of the supercapacitor before 6000 cycles shown by intersecting with the real axis in the high frequency region is almost the same as the Rs after 6000 cycles. All of these data prove that the electrode materials grown on carbon cloth have excellent performance. The Rf is calculated at about 1.4 Ω and 1.5 Ω before and after 6000 cycles, respectively.
Based on all of the above results, the β-NiMoO4 NW arrays on a carbon cloth electrode are promising for supercapacitor applications. The high performance of the β-NiMoO4 NW array electrode can be listed as follows. First, as shown in Fig. 9(a) and (b), the poor crystallinity which can provide more mesopores and the open space between NWs greatly increases conductivity through providing short transport and diffusion paths for both ions and electrons and could provide more electro-active sites for Li+.46,47 This is because the β-NiMoO4 NW arrays were synthesized at relatively low temperature and annealed in air. Second, the high capacitance of β-NiMoO4 NW arrays is mainly attributed to the deintercalation/intercalation of Li+ and the reversible redox reaction of Ni(II)/Ni(III).31,38,48 The possible reactions of the deintercalation/intercalation of Li+ and reversible redox reaction of Ni(II)/Ni(III) are listed as follows.
| NixMo1−xO4 + yLi+ + ye−1 ↔ LiyNixMo1−xO4 | (7) |
| NixMo1−xO4 + 3xLi+ + xe−1 ↔ Li3xMo1−xO4 + xNi2+ | (8) |
The β-NiMoO4 NW arrays have better conductivity than NiMoO4·xH2O nanowires, which is beneficial for the fast electron transfer.25,49 Third, from Fig. 9(c), we can see that the asymmetrical supercapacitor consists of a carbon rod, electrolyte, separator and β-NiMoO4 NW arrays supported on carbon cloth. And the carbon rod has high conductivity and environmental friendliness. Furthermore, the cylindrical supercapacitor can guarantee that all of the β-NiMoO4 NW arrays grown on carbon cloth are saturated enough and allow easy contact between ions and electrons. Last, the β-NiMoO4 NW arrays grown on carbon cloth can ensure good mechanical adhesion and enough contact between the carbon cloth and the β-NiMoO4 NW arrays. It is shown that it can be used to fabricate solid-state supercapacitor devices, and one hybrid device could light a light-emitting diode (LED) for 260 seconds, as shown in Fig. 9(d). The charging curve is shown in Fig. S3(d)† and the screen is displayed in S4.†
4. Conclusions
In summary, β-NiMoO4 NW arrays grown on carbon cloth have been synthesized through a hydrothermal method. This material is characterized by XRD, EDS, BET, SEM and TEM. An asymmetrical cylindrical solid supercapacitor was fabricated. This structure shows outstanding electrochemical performance with a largest specific capacitance of 414.7 F g−1 at a current density of 0.25 A g−1, a highest energy density of 36.86 W h kg−1 at a power density of 100 W kg−1 and a maximum power density of 1100 W kg−1 at an energy density of 12.3 W h kg−1. It retains 65.9% of its initial capacitance after 6000 cycles. It is demonstrated that it can be used to fabricate a solid-state supercapacitor device, and one hybrid device could light a light-emitting diode (LED) for 260 seconds. The whole evidence proves that the β-NiMoO4 nanostructure has high potential for the next generation of high performance electrochemical supercapacitors.Acknowledgements
This work has been funded by the NSFC (11204388), the SRFDP (20120191120039), the NSFCQ (cstc2014jcyjA50030), the Development Program (“863” Program) of China (2015AA034801), the Fundamental Research Funds for the Central Universities (No. CQDXWL-2014-001, No. CQDXWL-2013-012, No. 106112015CDJXY300004), and the large-scale equipment sharing fund of Chongqing University.References
- P. Yang and W. J. Mai, Nano Energy, 2014, 8, 274 CrossRef CAS.
- S. G. Dai, Y. Xi, C. G. Hu, B. S. Hu, X. L. Yue, L. Cheng and G. Wang, RSC Adv., 2014, 4, 40542 RSC.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867 CAS.
- S. E. Moosavifard, J. Shamsi, S. Fani and S. Kadkhodazade, RSC Adv., 2014, 4, 52555 RSC.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- S. E. Moosavifard, J. Shamsi and M. Ayazpour, Ceram. Int., 2015, 41, 1831 CrossRef CAS.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 11, 845 CrossRef PubMed.
- H. Jiang, P. S. Lee and C. Li, Energy Environ. Sci., 2013, 6, 41 CAS.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 CAS.
- J. Bae, M. K. Song, Y. J. Park, J. M. Kim, M. Liu and Z. L. Wang, Angew. Chem., Int. Ed., 2011, 50, 1683 CrossRef CAS PubMed.
- Z. Weng, Y. Su, D. W. Wang, F. Li, J. Du and H. M. Cheng, Adv. Energy Mater., 2011, 1, 917 CrossRef CAS.
- V. Khomenko, E. Raymundo-Pinero and F. Beguin, J. Power Sources, 2006, 153, 183 CrossRef CAS.
- L. Demarconnay, E. Raymundo-Pinero and F. Beguin, J. Power Sources, 2011, 196, 580 CrossRef CAS.
- C. Yuan, J. Li, L. Hou, L. Yang, L. Shen and X. Zhang, Electrochim. Acta, 2012, 78, 532 CrossRef CAS.
- F. Zhang, C. Yuan, X. Lu, L. Zhang, Q. Che and X. Zhang, J. Power Sources, 2012, 203, 250 CrossRef CAS.
- M. J. Deng, J. K. Chang, C. C. Wang, K. W. Chen, C. M. Lin, M. T. Tang, J. M. Chen and K. T. Lu, Energy Environ. Sci., 2011, 4, 3942 CAS.
- R. Kotz and M. Chalen, Electrochim. Acta, 2000, 45, 2482 CrossRef.
- Q. Wang, X. Wang, B. Liu, G. Yu, X. Hou, D. Chen and G. Shen, J. Mater. Chem. A, 2013, 1, 2468 CAS.
- D. P. Cai, B. Liu, D. D. Wang, Y. Liu, L. L. Wang, L. Han and Y. R. Wang, Electrochim. Acta, 2014, 125, 294 CrossRef CAS.
- M. C. Liu, L. B. Kong, C. Lu, X. M. Li, Y. C. Luo and L. Kang, Mater. Lett., 2013, 94, 197 CrossRef CAS.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- L. Yu, G. Zhang, C. Yuan and X. W. Lou, Chem. Commun., 2013, 49, 137 RSC.
- S. G. Dai, Y. Xi, C. G. Hu, X. L. Yue, L. Cheng and G. Wang, J. Power Sources, 2015, 274, 477 CrossRef CAS.
- B. Moreno, E. Chinarro, M. T. Colomer and J. R. Jurado, J. Phys. Chem. C, 2010, 114, 4251 CAS.
- J. Haetge, I. Djerdj and T. Brezesinski, Chem. Commun., 2012, 48, 6726 RSC.
- J. H. Ahn, G. D. Park, Y. C. Kang and J. H. Lee, Electrochim. Acta, 2015, 174, 102 CrossRef CAS.
- B. H. Qu, Y. J. Chen, M. Zhang, L. L. Hu, D. N. Lei, B. G. Lu, Q. H. Li, Y. G. Wang, L. B. Chen and T. H. Wang, Nanoscale, 2012, 4, 7810 RSC.
- D. Guo, Y. Z. Luo, X. Z. Yu, Q. H. Li and T. H. Wang, Nano Energy, 2014, 8, 174 CrossRef CAS.
- J. Yan, Z. J. Fan, W. Sun, G. Q. Ning, T. Wei, Q. Zhang, R. F. Zhang, L. J. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632 CrossRef CAS.
- A. L. M. Reddy, F. E. Amitha, I. Jafri and S. Ramaprabhu, Nanoscale Res. Lett., 2008, 3, 145 CrossRef.
- A. S. Adekunle, B. O. Agboola, K. I. Ozoemena, E. E. Ebenso, J. A. O. Oyekunle, O. S. Oluwatobi and J. N. Lekitima, Int. J. Electrochem. Sci., 2015, 10, 3414 CAS.
- G. K. Veerasubramani, K. Krishnamoorthy, R. Sivaprakasam and S. J. Kim, Mater. Chem. Phys., 2014, 147, 836 CrossRef CAS.
- T. Yang, H. Zhang, Y. Z. Luo, L. Mei, D. Guo and Q. L. Li, Electrochim. Acta, 2015, 158, 327 CrossRef CAS.
- A. Klein, P. Axmann, C. Yada and M. W. Mehrens, J. Power Sources, 2015, 288, 302 CrossRef CAS.
- M. C. Liu, L. Kang, L. B. Kong, C. Lu, X. J. Ma, X. M. Li and Y. C. Luo, RSC Adv., 2013, 3, 6472 RSC.
- D. P. Cai, D. D. Wang, B. Liu, Y. R. Wang, Y. Liu, L. L. Wang, H. Li, H. Huang, Q. H. Li and T. H. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12905 CAS.
- W. Hong, J. Q. Wang, P. W. Gong, J. F. Sun, L. Y. Niu, Z. G. Yang, Z. F. Wang and S. R. Yang, J. Power Sources, 2014, 270, 516 CrossRef CAS.
- L. Y. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. J. Dai, F. C. Feng, B. Hu, X. Wang, L. Gong, J. Chen, C. G. Hu, Y. X. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2012, 6, 656 CrossRef CAS PubMed.
- M. C. Liu, L. Kang, L. B. Kong, C. Lu, X. J. Ma, X. M. Li and Y. C. Luo, RSC Adv., 2013, 3, 6472 RSC.
- Q. Xue, Z. Xie, L. S. Zhang, X. L. Ma, X. L. Wu, Y. G. Guo, W. G. Song, Z. B. Li and T. B. Cao, Nanoscale, 2011, 3, 2703 RSC.
- R. S. Ray, B. Sarma and M. Misra, Mater. Lett., 2015, 155, 102 CrossRef CAS.
- B. M. Sanchez, T. Brousee, C. R. Castro, V. Nicolosi and P. S. Grant, Electrochim. Acta, 2013, 91, 253 CrossRef.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- S. Chen, W. Xing, J. Duan, X. Hu and S. Z. Qian, J. Mater. Chem. A, 2013, 1, 2941 CAS.
- K. K. Purushothaman, M. Cuba and G. Muralidharan, Mater. Res. Bull., 2012, 47, 3348 CrossRef CAS.
- B. Moreno, E. Chinarro, M. T. Colomer and J. R. Jurado, J. Phys. Chem. C, 2010, 114, 4251 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14704b |
| This journal is © The Royal Society of Chemistry 2015 |