Recyclable ionic liquid iodinating reagent for solvent free, regioselective iodination of activated aromatic and heteroaromatic amines†
Abstract
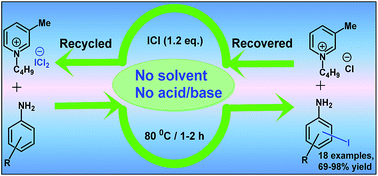
This article describes a simple, efficient method for iodination of activated aromatic and heteroaromatic amines using recyclable 1-butyl-3-methylpyridinium dichloroiodate (BMPDCI) as an ionic liquid iodinating reagent, in the absence of any solvent. The main advantages are a simple efficient procedure, good yields and no need for any base/toxic heavy metals, or oxidizing agents. The ionic liquid was recovered and recycled in five subsequent reactions, without much loss of activity. This method was applied for the synthesis of the antiprotozoal drug iodoquinol and the antifungal drug clioquinol.

 Please wait while we load your content...
Please wait while we load your content...