Enhancing the effect of bisulfite on sequestration of selenite by zerovalent iron†
Jinxiang Li‡
a,
Chao Wang‡a,
Junlian Qiao*a,
Hejie Qinab and
Lina Lib
aState Key Laboratory of Pollution Control and Resources Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, People's Republic of China. E-mail: qiaoqiao@tongji.edu.cn; ljx870616@126.com; wangchao962@163.com; chinhj@foxmail.com; Tel: +86-021-65489163
bShanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201204, People's Republic of China. E-mail: lilina@sinap.ac.cn
First published on 1st September 2015
Abstract
The enhancing effect of bisulfite (HSO3−) on the kinetics of Se(IV) sequestration by zerovalent iron (ZVI) was systematically investigated as a function of headspace volume, HSO3− concentration and initial pH (pHini). To exclude the role of HSO3− as an electrolyte, the kinetics of Se(IV) removal by ZVI with the presence of SO42− was determined as a control. With increasing headspace volume from 0 to 2.0 mL, the rate of Se(IV) removal by ZVI experienced a considerable enhancement whereas the further increase in the headspace volume resulted in a drop in Se(IV) removal rate. Se(IV) was always removed by ZVI with a higher rate in the presence of HSO3− than that in the presence of SO42− at various headspace volumes, which was mainly ascribed to the release of H+ and the depletion of O2 from the oxidation of HSO3− (i.e., 2HSO3− + O2 → 2SO42− + 2H+). Furthermore, HSO3− accelerated the reduction of ferric oxides and hydroxides to a Fe(II)-containing solid intermediate, which was beneficial to the reductive removal of Se(IV). The SEM, Fe K-edge XAFS and Se XANES analysis for Se(IV)-treated ZVI samples confirmed that HSO3− facilitated the transformation of ZVI to iron(oxyhydr)oxides (e.g., magnetite and lepidocrocite) and the reduction of Se(IV) to Se(0) compared to SO42−. The enhancing effect of HSO3− on Se(IV) sequestration varied with the concentration of HSO3− and initial pH, with the greatest effect achieved at 2.0 mM of Se(IV) and pHini 5.0. Since bisulfite is inexpensive and its final product is sulfate, a common anion existing in water, taking advantage of bisulfite to enhance the ZVI's reactivity under limited oxygenated conditions is a promising method.
1. Introduction
Zerovalent iron (ZVI), as a readily available, inexpensive, nontoxic and moderately strong reducing agent,1,2 has been successfully applied for the remediation/treatment of groundwater/wastewater.1,3,4 Nevertheless, owing to the inherent passive film, granular ZVI or iron filings have low intrinsic reactivity towards contaminants in laboratory studies and field demonstrations.1 Furthermore, the reactivity of ZVI decreased over time due to the precipitation of ferric(oxyhydr)oxides on the surface of iron.5 Considering that the low reactivity of ZVI has become a major concern in the ZVI-based systems, it is highly desirable to develop methods that can significantly meliorate the reactivity of ZVI for its further environmental application.1 Various countermeasures including acid washing,6 H2-reducing pretreatment,7 combination with sonication8 or weak magnetic field,1,4,9 and electrochemical reduction,10 as well as the synthesis of ZVI-based bimetals have been proposed to enhance the reactivity of ZVI.11 Nevertheless, the disadvantages of these methods should be addressed, such as relatively complex procedures, extra costs, and ecotoxicity.1,2The inherent passive film and subsequent authigenic mineral precipitation are undesirable in environmental applications since they may mask the redox active sites where exchange of electrons between ZVI and contaminant or reduce the barrier permeability by occupying available pore space.12 However, the FeII adsorbed to ferric oxides and hydroxides with higher reducing power (−0.35 to −0.65 V for Fe(s)III/Fe(s)II) than that of FeII/Fe0 (−0.44 V) has been reported to be beneficial for contaminants reduction.13,14 Recently, several studies have also confirmed that the Fe(II)-containing solid intermediate, e.g., Fe(II)Fe(III)hydroxide,13 green rust,15,16 magnetite,17 and pyrite,18 etc., can abiotically reduce contaminants whether the source of the FeII is bacteria-mediated regeneration,19–21 chemical reduction,22 or direct addition.23,24 Considering that the in situ generated Fe(III) can be rapidly reduced to Fe(II) by the inexpensive bisulfite (HSO3−) (eqn (1)), taking advantage of bisulfite to enhance the ZVI's reactivity may be achieved. However, this hypothesis has not been validated. On one hand, oxygen (O2) can be quenched by HSO3−, accompanied with the release of H+ via eqn (2).25,26 On the other hand, H+ and O2 had been reported to have great influence on the reactivity of ZVI toward contaminants.1,9,27–29 Consequently, the coupled influence of HSO3− and O2 on the reactivity of ZVI should be clarified.
| HSO3− + 2Fe3+ + H2O → 2Fe2+ + SO42− + 3H+ | (1) |
| 2HSO3− + O2 → 2SO42− + 2H+ | (2) |
The prevalence of selenium in water poses a potential toxicity or deficiency to humans, animals, and some plants within a very narrow concentration range.15,30 Selenium-contaminated waters mainly originate from crude oil processing in refinery operations, discharging agricultural drainage and treating mining wastewater.31,32 Selenium can exist in aquatic environments in several states of oxidation: selenide (Se(-II)), selenium (Se(0)), selenite (Se(IV)), and selenate (Se(VI)).33 The two oxidation states, Se(IV) and (Se(VI)), are highly soluble, thus bioavailable and potentially toxic, while the reduced forms (Se(-II) and Se(0)) are insoluble and correspondingly much less bioavailable.34 Moreover, the acute toxicity of Se(IV) is almost 10 times greater than that of Se(VI).35 In view of the redox-dependent solubility and toxicity of selenium, its oxyanions, particularly Se(IV), can precipitate as insoluble Se(0) or Se(-II) by reduction with ZVI or nanoscale ZVI,3,4,36–39 thereby creating an efficient sink for selenium.40,41 Therefore, Se(IV) was employed as a target contaminant to examine the influence of HSO3− on the reactivity (i.e., the reductive capability) of ZVI.
In sum, the main objectives of this study were to (1) investigate the effect of HSO3− on the kinetics of Se(IV) sequestration by ZVI in limited oxygenated water; (2) determine the role of HSO3− in enhancing the reactivity of ZVI toward Se(IV) removal; (3) evaluate the effects of HSO3− concentration and initial solution pH on Se(IV) removal by ZVI under limited oxygenated conditions.
2. Experimental section
2.1. Materials
All chemicals were of analytical grade and all solutions were prepared with Milli-Q water, unless otherwise specified. The ZVI used in this study was obtained from Sinopharm Chemical Reagent Co., Ltd., with a BET surface area of 0.15 m2 g−1. The SEM images, particle distribution and XRD pattern of the pristine ZVI sample employed in this study are presented in the ESI (Fig. S1†). All the other chemicals were purchased from Shanghai Qiangshun Chemical Reagent Company.2.2. Bach experiments
To investigate the influence of HSO3− on the reactivity of ZVI toward Se(IV) removal in limited oxygenated water, the batch reactor experiments were performed in 25 mL serum vials sealed with headspace containing air for 0–5.0 mL, which was injected by a syringe through the stopper. Prior to tests, reaction solution containing Se(IV) and background ions was freshly prepared by the N2-sparged water in an anoxic chamber, then the initial pH was adjusted by dropwise addition of a NaOH or H2SO4 solution. No measure was taken to maintain the pH constant during the reaction. In a typical experiment, the dosage of ZVI was 2.0 mM and the initial concentration of Se(IV) was 10.0 mg L−1 while the concentration of HSO3− varied from 0 to 2.5 mM. Before being sealed and subsequent air injection to initiate an experimental run, the dosing of iron powder was immediately accomplished. The vials were sealed with the Teflon-lined butyl rubber stoppers and placed on a rolling mixer (60 rpm) in a temperature-controlled chamber (∼25 °C). It should be specified that the bisulfite per se (without ZVI) could not remove Se(IV) (Fig. S2†) and reduce Se(IV) to Se(0) since no pink color resulting from Se(0) was observed. Additionally, to exclude the role of HSO3− as an electrolyte and the influence of generated SO42− on Se(IV) removal by ZVI, the experiments with SO42− at same molar concentration as HSO3− were carried out to work as the control.2.3. Chemical analysis and solid phase characterization
The sample in each reaction vial was sacrificed for analysis, which was collected at given time intervals using a 10 mL syringe and filtered immediately through a 0.22 μm membrane filter, then acidified for analysis. Concentration of the residual selenium in the filtrate was analyzed using a PF5 atomic fluorescence spectrometer (Beijing Purkinje General Instrument Co., Ltd.) and the Fe(II) concentration in solution was determined by the 1,10-phenanthroline colorimetric method using an UV-visible spectrophotometer at 510 nm.42Specific surface areas of the ZVI samples were determined by nitrogen adsorption using the BET method (Micrometrics ASAP 2020). Morphology of the pristine ZVI and the Se(IV)-treated ZVI samples was determined with Scanning Electron Microscopy (SEM) using a Hitachi 4700 microscope. The size distribution of the pristine ZVI particles was examined by a Bettersize 2000. The XRD pattern of the pristine ZVI was collected using a Rigaku DXR-8000 computer-automated diffractometer.
The reacted ZVI samples were collected on membrane filters (0.22 μm) and washed with deionized water in a nitrogen-filled glove box, and then freeze-dried under vacuum, and put them into zipper bags before being subjected to Fe K-edge and Se K-edge X-ray Absorption Fine Structure (XAFS) analysis. Particular care was taken to minimize the beam-induced oxidation of samples by placing the sample stands filled with reacted ZVI samples in a nitrogen-filled glove box for 6 hours before transferring them to zippered bags in this glove box. Negligible changes in the line-shape and peak position of Se K-edge X-ray absorption near edge structure (XANES) spectra were observed between two scans taken for a specific sample. The XAFS spectra were recorded at room temperature using a 4 channel Silicon Drift Detector (SDD) Bruker 5040 at beam line BL14W1 of the Shanghai Synchrotron Radiation Facility (SSRF), China. Fe and Se K-edge XAFS spectra were recorded in transmission mode and fluorescence mode using a Si(111) double crystal monochromator, respectively. The spectra were processed and analyzed by the software codes Athena.43 The oxidation states of selenium in solid phase were analyzed by linear combination fitting (LCF) using reference compounds of FeSe (Se(-II)), Se powder (Se(0)), Na2SeO3 (Se(IV)) and Na2SeO4 (Se(VI)). The major species of Fe in Se-treated ZVI corrosion products were also quantified by LCF using the collection of reference materials including metallic Fe (Fe0), wustite (FeO), maghemite (γ-Fe2O3), magnetite (Fe3O4), goethite (α-FeOOH) and lepidocrocite (γ-FeOOH).
3. Results and discussion
3.1. Oxygen effect on the reactivity of ZVI toward Se(IV) sequestration
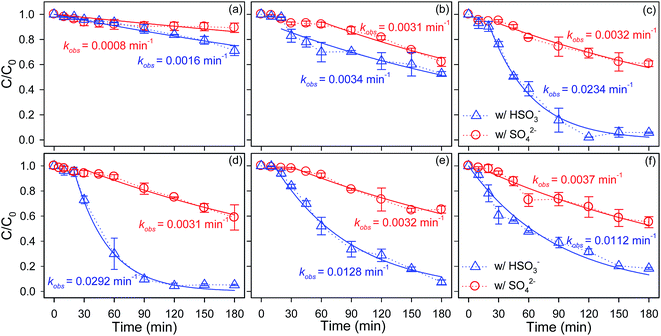
The influence of headspace volume on the kinetics of Se(IV) removal by ZVI was investigated, as depicted in Fig. 1. Obviously, headspace remarkably improved the reactivity of ZVI toward substrate regardless the presence of SO42− or HSO3−. Approximately 34.8–94.8% of Se(IV) was removed by ZVI in 180 min in limited oxygenated water (Vair = 0.5–5.0 mL), whereas only 10.7–28.9% of Se(IV) was sequestrated by ZVI within the same period but under anoxic condition. The O2-dependent characteristic of the ZVI's reactivity toward contaminants had also been reported by other researchers and was always explained by the promoted ZVI dissolution rate (eqn (3) and (4)) and/or the formation of the Fe(II)-solid intermediate and Fe(III) (oxyhydr)oxides on the ZVI surface providing reducing power and large surface area.13,27,44–46| 2Fe0 + O2 + 2H2O → 2Fe2+ + 4OH− | (3) |
| 2Fe0 + O2 + 4H+ → 2Fe2+ + 2H2O | (4) |
3.2. Coupled effects of O2 and HSO3− on the reactivity of ZVI toward Se(IV) sequestration
As illustrated in Fig. 1, a brief lag phase appeared in the kinetics of Se(IV) removal by ZVI in the presence of SO42− or HSO3−, both in this study and in our previous work.1,3,4,9,36 Analogous effects have been reported in previous investigations with a diversity of iron types and contaminant natures.4,47,48In the presence of SO42−, it was found that the lag period of Se(IV) removal by ZVI was gradually alleviated from 60 to 20 min with increasing the volume of headspace from 0.5 to 5.0 mL. The lag behavior in the initial period of Se(IV) removal by ZVI was mainly ascribed to the oxide film coated on the pristine ZVI particles inhibiting the mass transfer of substrate to the Fe0 surface.9 The other possible explanation for this transient behavior was that the O2 in headspace, which expedites the pitting of the passive film (eqn (3)–(6)), and thus, Se(IV) reduction by ZVI via eqn (7).
| Fe0 + 2H2O → Fe2+ + H2 + 2OH− | (5) |
| Fe0 + 2H+ → Fe2+ + H2 | (6) |
| 2Fe0 + HSeO3− + 5H+ → 2Fe2+ + Se0↓ + 3H2O | (7) |
After the lag period, the main portion of each data set can be reasonably well simulated by the pseudo-first-order rate law (eqn (8)).49
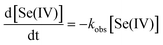 | (8) |
As for the kinetics of Se(IV) removal by ZVI in the presence of HSO3−, the lag behavior was susceptible to the headspace volume and the lag phase shortened from 20 to 0 min with increasing the volume of headspace from 0.5 to 5.0 mL. In addition, Fig. 1 and S4† revealed that the performances of ZVI for Se(IV) sequestration experienced a more dynamic and more variable scenario compared to that in the coexistence of headspace and SO42−, accompanied with a distinctively bell-shaped pattern associated with the headspace volume. The Se(IV) sequestration rate constant was enhanced progressively from 0.0016 to 0.0292 min−1 with increasing the headspace for containing air from 0 to 2.0 mL. With further increase in the headspace volume, the reactivity of ZVI reached a relatively recession. It should be emphasized that the ZVI kept a higher performance for removing Se(IV) in oxygenated water than that without headspace, which should be largely associated with the reduction of Fe(III)(oxyhydr)oxides to the Fe(II)-solid intermediate by HSO3− on the ZVI surface providing reducing power for Se(IV) following eqn (7) and (9).50,51
| 4Fe2+ + HSeO3− + 9H2O → 4Fe(OH)3 + Se0↓ + 7H+ | (9) |
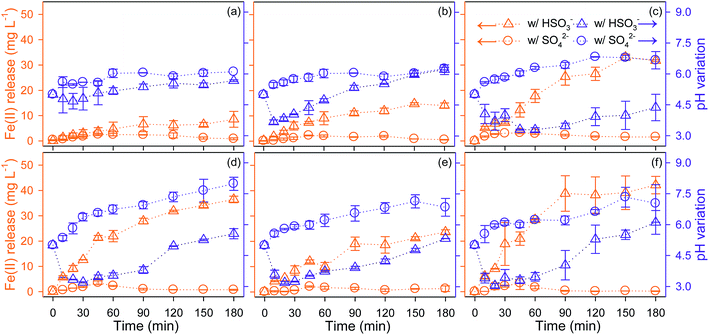
Fig. 1 demonstrates that the coupled effects of O2 and HSO3− resulted in faster selenium removal kinetics than that in the coexistence of O2 and SO42−, which were consistent with the variations of aqueous Fe(II) concentrations and solution pH during Se(IV) sequestration by ZVI in the presence of SO42−/HSO3− and O2, as shown in Fig. 2.
In the presence of SO42−, the concentration of Fe(II) increased progressively within 45 min and then dropped gradually in the process of Se(IV) removal by ZVI, which was accompanied with a mild increase of solution pH during the initial stage of reaction. Hereafter, pH was almost constant, which may be associated with the Fe2+ oxidation by O2 and Fe3+ hydrolysis (eqn (10) and (11)). Different from the case in the presence of SO42−, the solution pH during Se(IV) removal by ZVI in the presence of HSO3− dropped dramatically during the initial period of reaction (from 0 to 10 or 30 min, eqn (2)) and hereafter experienced a progressively elevated stage, accompanied with a rapid release of Fe(II) with prolonged reaction time. Moreover, Fe(III) can be reduced to Fe(II) by HSO3− via eqn (1), accompanied with the release SO42− and H+, which favors the corrosion of ZVI, accounting for the trend in the release of Fe(II).
| 4Fe2+ + O2 + 2H2O → 4Fe3+ + 4OH− | (10) |
| Fe3+ + 3H2O → 2Fe(OH)3 + 3H+ | (11) |
3.3. Iron corrosion and selenium speciation transformation in Se(IV)-treated ZVI samples
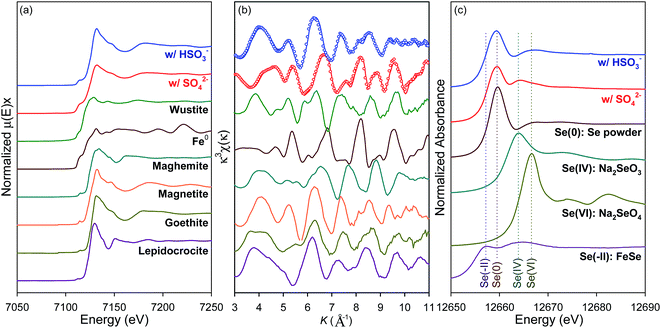
The ZVI corrosion behaviors with presence of O2 and HSO3− were further clarified by characterizing the Se(IV)-treated ZVI samples. The pristine ZVI used in this study was consisted of relatively smooth spheres (Fig. S1(a)†) and became slightly coarse during reacted with Se(IV) in the presence of SO42− for 30 min, 60 min and 180 min, respectively (Fig. S5†). Comparably, the Se(IV)-treated ZVI particles in the presence of HSO3− became more cracked with angular-shaped and platy structures (Fig. S5†), implying the more extensive corrosion upon the introduction of HSO3−. Moreover, the Fe K-edge XANES spectra and k3-weighted EXAFS spectra of these two Se(IV)-treated ZVI samples and the reference materials were shown in Fig. 3(a) and (b). In the presence of SO42−, the XANES and EXAFS spectra of Se(IV)-treated ZVI sample was more analogous to that of the Fe0 than that with the presence of HSO3− (Fig. 3(a)). Meanwhile, the latter sample was more analogous to those of the Fe(II)/Fe(III) reference compounds (Fig. 3(a)), implying that these solids were mainly composed of nonmetallic Fe. To identify the composition of corrosion products, linear combination fitting (LCF) analysis was carried out based on the Fe k3-weighted EXAFS spectra (Fig. 3(b)) and the corresponding fit results were summarized in Table S1.† In the presence of SO42−, the Se(IV)-treated ZVI sample was consist of magnetite (43.0%), wustite (20.4%), ferrihydrite (9.5%), lepidocrocite (8.0%), and some metallic Fe0 (19.1%). Then SO42− was replaced with HSO3−, the fraction of Fe0 in the Se(IV)-treated ZVI sample decreased greatly to 5.4% and ZVI corrosion was considerably enhanced with the major corrosion products being magnetite (57.7%) and lepidocrocite (19.4%), along with some wustite (8.2%) and maghemite (9.3%). It was generally acknowledged that the very high conductivity of magnetite with almost metallic character was beneficial for the electron transport and the loose and porous structure of lepidocrocite favored the mass transport between the solid phase and aqueous phase.52,53 Therefore, it could be inferred that the appreciable reactivity of ZVI was mainly associated with the involvement of magnetite and lepidocrocite.To further explore the coupled effects of O2–HSO3− on the reactivity of ZVI toward Se(IV) removal, the selenium speciation in the corresponded precipitates collected at 180 min was analyzed with XANES, as demonstrated in Fig. 3(c). The Se K-edge XANES data for several Se reference compounds including FeSe (Se(-II)), selenium powder (Se(0)), sodium selenite (Se(IV)), and sodium selenate (Se(VI)) were analyzed to establish the reference X-ray absorption K-edge energies (E0).46,54 The XANES spectrum of Se(IV)-treated ZVI in the presence of O2 and HSO3− demonstrated that Se(IV) removal by ZVI was mainly achieved via reduction following eqn (7) and (9). However, only partial of the removed Se(IV) was reduced to Se(0) when SO42− was employed as background electrolyte. The transient Se(IV) adsorption step on the reductive surface forming the shell surrounding the iron core should be necessary for reduction of dissolved Se(IV) by ZVI and adsorbed Fe(II).1,3,55 Therefore, it could be concluded that the coupled presence of O2 and HSO3− was more beneficial for the reduction of Se(IV) to Se(0) as compared to that by the synergetic effects of O2 and SO42−.
3.4. Effects of SO42−/HSO3− concentration and pH on Se(IV) sequestration by ZVI
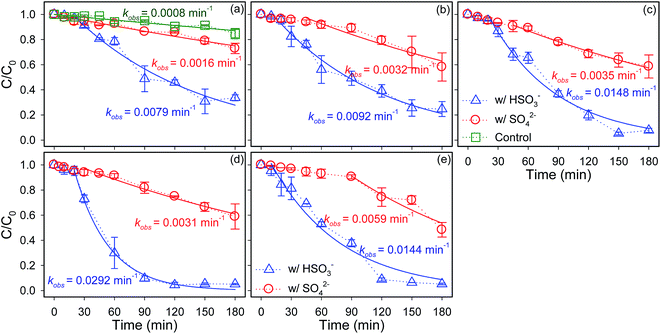
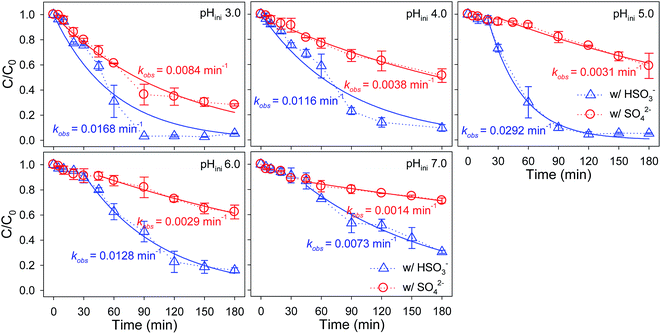
The influence of SO42−/HSO3− concentrations on the kinetics of Se(IV) sequestration by ZVI in limited oxygenated water at pHini 5.0 were demonstrated in Fig. 4. At various SO42−/HSO3− concentrations, ZVI always removed Se(IV) at higher rate constants in the presence of HSO3− than those in the presence of SO42−. In addition, the kinetics of selenium disappearance could be roughly divided into two stages, a lag period followed by a rapid removal period, which was simulated with pseudo-first order model (eqn (8)). It was found that the rate constants of Se(IV) removal by ZVI were increased appreciably from 0.0008 to 0.0059 min−1 whereas did not mediate the lag period with increasing the concentration of SO42− from 0 to 2.5 mM, implying that the iron oxides coated on the pristine ZVI particles could weaken the enhancement for Se(IV) removal by SO42−. On the contrary, after the introduction of HSO3−, the lag phase in the initial period of Se(IV) removal by ZVI was shortened from 20 to 10 min with increasing the HSO3− concentration from 0.5 to 2.5 mM. Moreover, the rate constants of Se(IV) sequestration by ZVI were enhanced progressively from 0.0079 to 0.0292 min−1 as the concentration of HSO3− was elevated from 0.5 to 2.0 mM. However, as the concentration of HSO3− was further increased to 2.5 mM, the removal rate of Se(IV) decreased to 0.0144 min−1. The influence of HSO3− concentration on the reactivity of ZVI should be mainly associated with the H+ release and the O2 depletion from the oxidation of HSO3− following eqn (2). With increasing the HSO3− concentration, the progressive enhancements should be likely due to the decline of solution pH and the depletion of O2 preventing the passivation of ZVI. However, HSO3− of high concentration will quench O2, which will inhibit the formation of the Fe(III)(oxyhydr)oxides co-precipitating with Se(IV) and the galvanic corrosion of ZVI, and thus, Se(IV) reduction by ZVI via eqn (7).The effects of SO42−/HSO3− on Se(IV) removal by ZVI in limited oxygenated water over the pHini of 3.0–7.0 were also evaluated, as depicted in Fig. 5. HSeO3− is the major specie of the aqueous Se(IV) at pH 3.0–7.0 since the pKa1 and pKa2 of H2SeO3 are 2.62 and 8.23, respectively (Fig. S6†).56 Thus, the synergetic effects of O2 and pHini on Se(IV) removal should be rarely ascribed to the species variation of Se(IV).1 Regardless the introduction of SO42− or HSO3−, the removal of Se(IV) by ZVI exhibited self-acceleration characteristics, which involved a lag period and hereafter a rapid removal period. The duration of lag period extended gradually with increasing the pHini, verifying that the lower pH facilitated the abrasion of passive iron oxide layer and thus shortened the lag period.1,2,9 Beyond the lag period, it was additionally found that the pseudo-first-order rate constants (kobs) for Se(IV) removal in the presence of SO42− were dampen dramatically from 0.0084 to 0.0014 min−1 with increasing the solution pHini. Alternatively, the introduction of HSO3− markedly accelerated Se(IV) removal by ZVI and the kobs were 0.0168, 0.0116, 0.0292, 0.0128 and 0.0073 min−1 at pHini 3.0, 4.0, 5.0, 6.0 and 7.0, respectively. The best performance of ZVI for Se(IV) removal in the presence of HSO3− was achieved at pHini 5.0. It was well known that the adsorbed Fe(II) on ZVI surface contributed to the reductive removal of Se(IV).3 With increasing pHini, the reductive ability of Fe(II) increased while the amount of released Fe(II) dropped (Fig. S7†),57 and thus the most efficient Se(IV) removal was observed at pHini 5.0.
4. Conclusions
The bisulfite induced a significant enhancement on the kinetics of Se(IV) removal by ZVI and the improvement should be mainly ascribed to the release of H+ and the depletion of O2 arising from the oxidation of HSO3−. In addition, HSO3− facilitated the reduction of ferric(oxyhydr)oxides to Fe(II)-containing solid, which was beneficial to the reductive removal of Se(IV). Over the HSO3− concentration of 0–2.5 mM or pHini range of 3.0–7.0, ZVI could always keep higher reactivity toward Se(IV) sequestration in the presence of HSO3− than that in the presence of SO42−. The coupled effects of O2 and HSO3− on ZVI's reactivity toward Se(IV) removal experienced a more dynamic and more variable scenario (bell-shaped) than that in the presence of O2 and SO42−. Furthermore, the SEM, Fe K-edge XAFS and Se XANES analysis for Se(IV)-treated ZVI samples unraveled that binding O2 and HSO3− favored the transformation of ZVI to iron(oxyhydr)oxides (e.g., magnetite and lepidocrocite) and the reduction of Se(IV) to Se(0). Compared to the previous strategies for maintaining or enhancing the reactivity of ZVI, applying bisulfite will provide a promising alternative to improve the performance of the ZVI-based technology for environmental application, since the bisulfite is efficient, inexpensive and its final product is harmless. However, more efforts should be taken to evaluate this technology more thoroughly on field-scale abatement practices.Acknowledgements
This work was supported by the youth project of National Natural Science Fund (Grant 11405256) and Shanghai Municipal Natural Science Foundation (Grant 13ZR1447800). The authors thank the beamline BL14W1 (Shanghai Synchrotron Radiation Facility) for providing the beam time.References
- L. Liang, W. Sun, X. Guan, Y. Huang, W. Choi, H. Bao, L. Li and Z. Jiang, Water Res., 2014, 49, 371–380 CrossRef CAS PubMed.
- X. Guan, Y. Sun, H. Qin, J. Li, I. M. Lo, D. He and H. Dong, Water Res., 2015, 75, 224–248 CrossRef CAS PubMed.
- L. Liang, W. Yang, X. Guan, J. Li, Z. Xu, J. Wu, Y. Huang and X. Zhang, Water Res., 2013, 47, 5846–5855 CrossRef CAS PubMed.
- L. P. Liang, X. H. Guan, Z. Shi, J. L. Li, Y. N. Wu and P. G. Tratnyek, Environ. Sci. Technol., 2014, 48, 6326–6334 CrossRef CAS PubMed.
- C. B. Wang and W. X. Zhang, Environ. Sci. Technol., 1997, 31, 2154–2156 CrossRef CAS.
- K. C. K. Lai and I. M. C. Lo, Environ. Sci. Technol., 2008, 42, 1238–1244 CrossRef CAS.
- Y. H. Liou, S. L. Lo, C. J. Lin, W. H. Kuan and S. C. Weng, J. Hazard. Mater., 2005, 126, 189–194 CrossRef CAS PubMed.
- C. L. Geiger, C. A. Clausen, D. R. Reinhart, C. M. Clausen, N. Ruiz and J. Quinn, ACS Symp. Ser., 2003, 837, 286–303 CrossRef CAS PubMed.
- Y. K. Sun, X. H. Guan, J. M. Wang, X. G. Meng, C. H. Xu and G. M. Zhou, Environ. Sci. Technol., 2014, 48, 6850–6858 CrossRef CAS PubMed.
- L. Chen, S. Jin, P. H. Fallgren, N. G. Swoboda-Colberg, F. Liu and P. J. S. Colberg, J. Hazard. Mater., 2012, 239, 265–269 CrossRef PubMed.
- B. W. Zhu and T. T. Lim, Environ. Sci. Technol., 2007, 41, 7523–7529 CrossRef CAS.
- Y. Furukawa, J.-W. Kim, J. Watkins and R. T. Wilkin, Environ. Sci. Technol., 2002, 36, 5469–5475 CrossRef CAS.
- A. C. Scheinost and L. Charlet, Environ. Sci. Technol., 2008, 42, 1984–1989 CrossRef CAS.
- A. F. White and M. L. Peterson, Geochim. Cosmochim. Acta, 1996, 60, 3799–3814 CrossRef CAS.
- S. C. B. Myneni, T. K. Tokunaga and G. E. Brown, Science, 1997, 278, 1106–1109 CrossRef CAS.
- A. M. Scheidegger, D. Grolimund, D. Cui, J. Devoy, K. Spahiu, P. Wersin, I. Bonhoure and M. Janousch, J. Phys. IV, 2003, 104, 417–420 CAS.
- C. A. Gorski, J. T. Nurmi, P. G. Tratnyek, T. B. Hofstetter and M. M. Scherer, Environ. Sci. Technol., 2010, 44, 55–60 CrossRef CAS PubMed.
- C. Bruggeman, A. Maes, J. Vancluysen and P. Vandenmussele, Environ. Pollut., 2005, 137, 209–221 CrossRef CAS PubMed.
- M. L. McCormick, E. J. Bouwer and P. Adriaens, Environ. Sci. Technol., 2002, 36, 403–410 CrossRef CAS.
- H. Y. Shin, N. Singhal and J. W. Park, Chemosphere, 2007, 68, 1129–1134 CrossRef CAS PubMed.
- B. Lai, Y. X. Zhou, P. Yang, J. L. Wang, J. H. Yang and H. Q. Li, J. Hazard. Mater., 2012, 241, 241–251 CrossRef PubMed.
- S. Kim and F. W. Picardal, Environ. Toxicol. Chem., 1999, 18, 2142–2150 CrossRef CAS PubMed.
- R. A. Maithreepala and R. A. Doong, Environ. Sci. Technol., 2004, 38, 260–268 CrossRef CAS.
- J. E. Amonette, D. J. Workman, D. W. Kennedy, J. S. Fruchter and Y. A. Gorby, Environ. Sci. Technol., 2000, 34, 4606–4613 CrossRef CAS.
- R. E. Connick, Y. X. Zhang, S. Y. Lee, R. Adamic and P. Chieng, Inorg. Chem., 1995, 34, 4543–4553 CrossRef CAS.
- R. E. Connick and Y. X. Zhang, Inorg. Chem., 1996, 35, 4613–4621 CrossRef CAS.
- L. F. Greenlee, J. D. Torrey, R. L. Amaro and J. M. Shaw, Environ. Sci. Technol., 2012, 46, 12913–12920 CrossRef CAS PubMed.
- P. Sarin, V. L. Snoeyink, J. Bebee, K. K. Jim, M. A. Beckett, W. M. Kriven and J. A. Clement, Water Res., 2004, 38, 1259–1269 CrossRef CAS PubMed.
- B. Gu, T. J. Phelps, L. Liang, M. J. Dickey, Y. Roh, B. L. Kinsall, A. V. Palumbo and G. K. Jacobs, Environ. Sci. Technol., 1999, 33, 2170–2177 CrossRef CAS.
- B. D. Gibson, D. W. Blowes, M. B. J. Lindsay and C. J. Ptacek, J. Hazard. Mater., 2012, 241, 92–100 CrossRef PubMed.
- Y. Q. Zhang and J. N. Moore, Environ. Sci. Technol., 1996, 30, 2613–2619 CrossRef CAS.
- X. G. Meng, S. Bang and G. P. Korfiatis, Water Res., 2002, 36, 3867–3873 CrossRef CAS.
- C. V. Putnis, F. Renard, H. E. King, G. Montes-Hernandez and E. Ruiz-Agudo, Environ. Sci. Technol., 2013, 47, 13469–13476 CrossRef CAS PubMed.
- Y. Q. Zhang, C. Amrhein, A. Chang and W. T. Frankenberger, Sci. Total Environ., 2008, 407, 89–96 CrossRef CAS PubMed.
- R. L. D. Loyo, S. I. Nikitenko, A. C. Scheinost and M. Simonoff, Environ. Sci. Technol., 2008, 42, 2451–2456 CrossRef.
- L. P. Liang, X. Jiang, W. J. Yang, Y. Y. Huang, X. H. Guan and L. N. Li, Desalin. Water Treat., 2015, 53, 2540–2548 CrossRef CAS PubMed.
- R. Liu and R. Lal, J. Nanotechnol., 2012, 2012, 1–18 Search PubMed.
- R. A. Crane and T. B. Scott, J. Hazard. Mater., 2012, 211, 112–125 CrossRef PubMed.
- L. Ling, B. C. Pan and W. X. Zhang, Water Res., 2015, 71, 274–281 CrossRef CAS PubMed.
- T. K. Tokunaga, G. E. Brown, I. J. Pickering, S. R. Sutton and S. Bait, Environ. Sci. Technol., 1997, 31, 1419–1425 CrossRef CAS.
- Y. Q. Zhang and J. N. Moore, Appl. Geochem., 1997, 12, 685–691 CrossRef CAS.
- Y. K. Sun, X. M. Xiong, G. M. Zhou, C. Y. Li and X. H. Guan, Sep. Purif. Technol., 2013, 115, 198–204 CrossRef CAS PubMed.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- S. Klas and D. W. Kirk, Sep. Purif. Technol., 2013, 116, 222–229 CrossRef CAS PubMed.
- S. Klas and D. W. Kirk, J. Hazard. Mater., 2013, 252, 77–82 CrossRef PubMed.
- I. H. Yoon, K. W. Kim, S. Bang and M. G. Kim, Appl. Catal., B, 2011, 104, 185–192 CrossRef CAS PubMed.
- X. Jiang, J. Qiao, I. M. C. Lo, L. Wang, X. Guan, Z. Lu, G. Zhou and C. Xu, J. Hazard. Mater., 2015, 283, 880–887 CrossRef CAS PubMed.
- R. Miehr, P. G. Tratnyek, J. Z. Bandstra, M. M. Scherer, M. J. Alowitz and E. J. Bylaska, Environ. Sci. Technol., 2004, 38, 139–147 CrossRef CAS.
- T. L. Johnson, M. M. Scherer and P. G. Tratnyek, Environ. Sci. Technol., 1996, 30, 2634–2640 CrossRef CAS.
- L. Xie and C. Shang, Chemosphere, 2007, 66, 1652–1659 CrossRef CAS PubMed.
- P. Westerhoff and J. James, Water Res., 2003, 37, 1818–1830 CrossRef CAS.
- F. D. Coelho, J. D. Ardisson, F. C. C. Moura, R. M. Lago, E. Murad and J. D. Fabris, Chemosphere, 2008, 71, 90–96 CrossRef PubMed.
- A. Neumann, R. Kaegi, A. Voegelin, A. Hussam, A. K. M. Munir and S. J. Hug, Environ. Sci. Technol., 2013, 47, 4544–4554 CrossRef CAS PubMed.
- J. T. Olegario, N. Yee, M. Miller, J. Sczepaniak and B. Manning, J. Nanopart. Res., 2010, 12, 2057–2068 CrossRef CAS.
- Y. Q. Zhang, J. F. Wang, C. Amrhein and W. T. Frankenberger, J. Environ. Qual., 2005, 34, 487–495 CrossRef CAS.
- T. Z. Su, X. H. Guan, G. W. Gu and J. M. Wang, J. Colloid Interface Sci., 2008, 326, 347–353 CrossRef CAS PubMed.
- X. H. Guan, H. R. Dong, J. Ma, I. M. C. Lo and X. M. Dou, Sep. Purif. Technol., 2011, 80, 179–185 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14659c |
| ‡ Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |