DOI:
10.1039/C5RA14621F
(Paper)
RSC Adv., 2015,
5, 74438-74446
Synthesis of tetra-substituted olefins via annulation by Pd-catalyzed carbopalladation/C–H activation and solid state fluorescence properties†
Received
23rd July 2015
, Accepted 21st August 2015
First published on 24th August 2015
Introduction
The development of a new synthetic approach that enables the construction of complex molecules via a multiple bond forming process in a single operation with profound application of medicinal and material chemistry, enabling green and efficient organic synthesis, is a key motive of modern organic chemistry. In recent decades, transition-metal catalyzed functionalization of alkynes via C–C coupling has become a powerful strategy for the construction of basic to complex heterocyclic scaffolds.1 In this regard, palladium-catalyzed C–C coupling of alkynes is finding extensive utility through the formation of a carbopalladation adduct as a key intermediate.2,3 In addition to that, palladium-catalyzed and norbornene-mediated C–H bond activation and subsequent functionalization with various coupling partners, leading to the formation of the C–C bond offers unprecedented opportunities for the synthesis of polyfunctionalized aromatic compounds.4 This strategy has been extensively used to synthesize various natural products and bioactive compounds from easily accessible starting materials.5 In recent times, norbornenes are receiving increased attention for their participation in metal-catalyzed carbocyclic and C–H bond functionalization processes.6–8 We are motivated to explore palladium-catalyzed annulation of alkynes with norbornene via the C–H bond functionalization process.
Design and synthesis of solid state organic luminescent materials is attracting the attention of chemists and has thereby found profound importance in materials science and in the field of biology.9–12 Organic materials exhibiting fluorescence in the condensed phase are crucial topics in optoelectronic devices such as organic light-emitting diodes (OLEDs),10 fluorescent chemo/biosensors and as bio-imaging probes.11,12 Many organic luminophores are strongly emissive in solution but weakened or non-emissive in the solid or aggregate state, which is notoriously known as aggregation caused quenching (ACQ) in the condensed phase. Another photophysical phenomenon connected to luminogen aggregation is aggregation induced emission (AIE). Such types of molecules are nonemissive in the solution state but become strongly emissive upon aggregation.
Previously, we described the synthesis of tetra-substituted olefin based xanthene derivatives via palladium catalyzed intramolecular carbocyclization of substituted 2-bromobenzyl-N-propargylamines and their photophysical properties in both solid and aggregation states.13 As part of our ongoing efforts on the palladium catalyzed formation of tetra-substituted alkenes through cascade reactions,14 herein we report the palladium-catalyzed intermolecular annulation of 2-bromo-N-benzylpropargylamines with norbornene towards the formation of tetra-substituted helical olefin incorporated 1,2,3,4-tetrahydroisoquinoline and methanofluoren-9-ylidene (Scheme 1). In this domino process, suitably positioned internal aryl bromides trigger initiation of the cyclization, leading to the formation of one C(sp2)–C(sp2) and two C(sp3)–C(sp2) bonds, resulting in simultaneous creation of highly crowded tetra-substituted helical olefins.
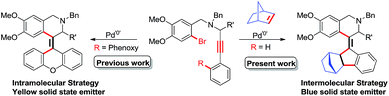 |
| | Scheme 1 Palladium-catalyzed synthesis of tetra-substituted olefins via a C–H functionalization process. | |
Results and discussion
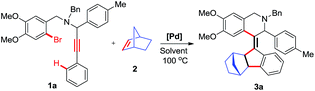
We started our initial investigation using N-benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-3-phenyl-1-(p-tolyl)prop-2-yn-1-amine (1a) as a model substrate for the formation of norbornene functionalized olefin 3a by adding 10 mol% of Pd(PPh3)4, 1.2 equiv. of norbornene and 3 equiv. of K2CO3 in DMF at 100 °C under N2. The desired cyclized product 3a was isolated in 46% yield (Table 1, entry 1). Interestingly, excessive addition of norbornene (2 equiv.) to the reaction mixture showed improvement in the yield of the product (Table 1, entry 2). However, similar efficiency was detected by further increasing the quantity of norbornene (Table 1, entry 3). In order to increase the yield of the product, we screened the reaction conditions by varying the bases and solvents (Table 1, entry 4–9). When we switched the catalyst from Pd(PPh3)4 to Pd(OAc)2 or reduced the catalyst to 5 mol% of Pd(PPh3)4, the desired cyclized product 3a was obtained in decreased yield (Table 1, entries 10 & 11). From the different set of experimental conditions, we concluded that the optimum reaction conditions are 10 mol% of Pd(PPh3)4, 2 equiv. norbornene and 3 equiv. K2CO3 in DMF as a solvent at 100 °C under N2. The structure of product 3a was deduced from 1H, 13C NMR and HRMS analysis. Furthermore, the connectivity and molecular structure of cyclic compounds were unambiguously confirmed by single crystal X-ray analysis of compound 3a (Fig. 1d).
Table 1 Optimization of reaction conditionsa
|
| Entry |
Catalyst (10 mol%) |
2 (equiv.) |
Base (equiv.) |
Solvent |
Timeb (h) |
Yieldc (%) |
| Reactions were carried out with 1a (0.10 mmol), catalyst (10 mol%), norbornene 2, base (3 equiv.) in anhydrous solvent (3 mL) at 100 °C under N2. Reactions monitored by TLC and based on complete consumption of 1a. Yield of isolated product after column chromatography. 20 mol% of PPh3 was used. 5 mol% of Pd(PPh3)4 was used. |
| 1 |
Pd(PPh3)4 |
1.2 |
K2CO3 |
DMF |
3.0 |
46 |
| 2 |
Pd(PPh3)4 |
2.0 |
K2CO3 |
DMF |
3.0 |
64 |
| 3 |
Pd(PPh3)4 |
3.0 |
K2CO3 |
DMF |
3.0 |
64 |
| 4 |
Pd(PPh3)4 |
2.0 |
Cs2CO3 |
DMF |
12 |
32 |
| 5 |
Pd(PPh3)4 |
2.0 |
Na2CO3 |
DMF |
12 |
28 |
| 6 |
Pd(PPh3)4 |
2.0 |
K3PO4 |
DMF |
12 |
32 |
| 7 |
Pd(PPh3)4 |
2.0 |
K2CO3 |
CH3CN |
12 |
38 |
| 8 |
Pd(PPh3)4 |
2.0 |
K2CO3 |
1,4-Dioxane |
12 |
30 |
| 9 |
Pd(PPh3)4 |
2.0 |
K2CO3 |
Toluene |
12 |
37 |
| 10 |
Pd(OAc)2 |
2.0 |
K2CO3 |
DMF |
12 |
42d |
| 11 |
Pd(PPh3)4 |
2.0 |
K2CO3 |
DMF |
7.0 |
36e |
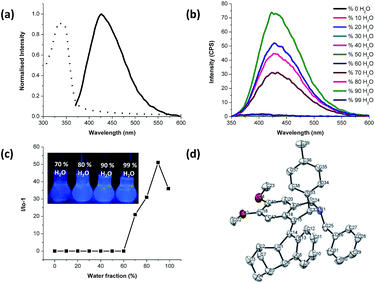 |
| | Fig. 1 (a) Normalized absorption (dash line) and PL (solid line) spectrum of compound 3a in thin film. (b) PL spectra of 3a in different H2O/CH3CN (v/v) mixtures; concentration 10 μM, excitation wavelength 320 nm. (c) Plot of PL peak intensity of 3a vs. water fractions in H2O/CH3CN mixtures. I0 was the PL intensity in pure CH3CN solution. The inset in panel c: photos of 3a in H2O/CH3CN mixtures (fw = 70, 80, 90 and 90%) taken under UV luminescence. (d) Ortep diagram of 3a (hydrogen atoms are omitted for clarity). | |
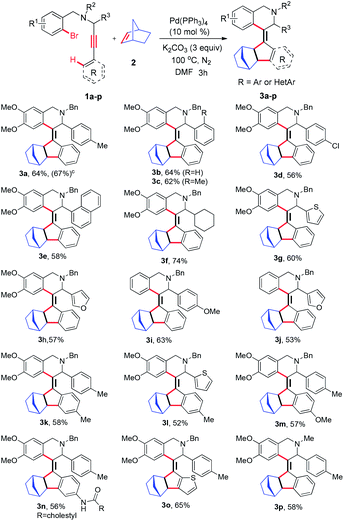
With the optimized conditions in hand, we next investigated the substrate scope of the annulation process using the designed propargylamines (1a–p), which were synthesized through A3 coupling reactions of various benzyl amines, aldehydes and terminal alkynes.15 All the substrates were subjected to optimized conditions; the reactions were well tolerated by a variety of functional groups and the cyclized olefinic compounds were formed in moderate to good yields. The results are presented in Table 2. When phenyl, 2-methylphenyl and 4-chlorophenyl substituted propargylamines were subjected to the optimum conditions, the desired tetra-substituted olefins 3b–d were obtained in yields of range 56–64%. Similarly, naphthyl-substituted substrate 1e also participated in this reaction to afford 3e in 58% yield. In the case of the cyclohexyl ring in the propargylic position, the cyclization occurred efficiently to afford a slightly higher yield of 3f (74%). Heterocyclic moiety-incorporating propargylamines 1g and 1h were also tolerated by the annulation reaction and the corresponding cyclized tetra-substituted olefins 3g and 3h in 60% and 57%, respectively, were obtained. The influence of substitution on the phenyl ring of phenylacetylene was subsequently examined. Substituents at the para-position of phenylacetylene, regardless of its electronic nature (methyl, methoxy, and amide), had a negligible effect on the outcome of the reaction, providing norbornene functionalized fused olefins (3k–n) in moderate yields. However, the 2-thienyl alkyne ring underwent a cyclization process and generated thiophene functionalized olefin 3o in good yield. In addition, we also investigated unsubstituted aryl bromide (1i & 1j) and N-methyl substituted propargylamines 1p to obtain the corresponding annulated product under similar reaction conditions (Table 2, 3i, 3j & 3p).
Table 2 Synthesis of helical olefin incorporated 1,2,3,4-tetrahydroisoquinolinesa,b
| Reactions were carried out with 1a–p (0.10 mmol), norbornene 2 (2.0 equiv.), 10 mol% Pd(PPh3)4 and K2CO3 (3 equiv.) in 3 mL anhydrous DMF (99.8%) at 100 °C under N2 for 3 h. Reaction was carried out with 1.0 mmol 1a.c Yield of isolated products after column chromatography. |
 |
On the basis of the product formation 3, a possible reaction mechanism has been proposed in Scheme 2. Initially, the palladium(0) species undergoes an oxidative addition with the Ar–Br bond 1 to generate the aryl palladium(II) intermediate A. This intermediate A involves coordination or chelation with tethered alkyne B, followed by intramolecular carbopalladation onto the alkyne, enabling the formation of the first new C–C bond C. After the 6-exo-dig cyclization, vinylic palladium(II) intermediate C subsequently undergoes an intermolecular carbopalladation with norbornene to form complex D as a result of the second C–C bond formation. The complex D induces C–H functionalization on the adjacent aromatic rings, leading to the formation of complex E. This process may be believed to proceed by either direct C–H insertion or electrophilic aromatic substitution by attack of the aryl ring onto the palladium(II) species. Subsequently, reductive elimination of complex E occurs to afford 3 with the formation of the third C–C bond and regeneration of the palladium catalyst.
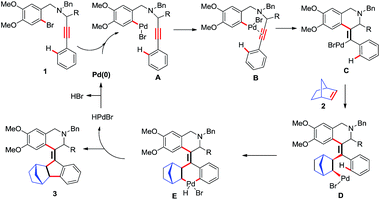 |
| | Scheme 2 Plausible mechanism for the cyclized product 3. | |
During the purification of 3a, we observed that the compound 3a did not show any emissive spot on the wet TLC plate under UV luminescence. After the evaporation of the solvent, a blue light-emitting spot became visible. Thus, the fluorescence was off and on in the wet and dry plates, respectively, implying that the synthesized compounds are nonemissive in solution but become luminescent when aggregated. To understand this intriguing visual observation, we measured the photoluminescence (PL) spectra of 3a in the thin film, which showed absorption and emission maxima at 346 and 427 nm, respectively (Fig. 1a). This unusual behavior of 3a is due to the phenomenon of aggregation induced emission (AIE) due to the complete restriction of rotational and vibrational motions in the condensed phase. In addition, to confirm the AIE nature of 3a, we measured the PL spectra of compound 3a by blending the acetonitrile solution of 3a with different ratios of water (Fig. 1b and c). The PL spectrum of 3a at a 10 μM solution in acetonitrile is nearly a flat line parallel to the abscissa. By slow addition of water into the solution, the value remained unchanged until water was increased to about fw ≤ 60%. However, in the ACN/water mixtures with high fractions of water (fw > 60%), compound 3a showed emission spectra with a clear peak, where the solvation power of the mixture was drastically reduced to such an extent that the luminogen molecules begin to aggregate. The PL spectrum showed maximum emission intensity at a water fraction of about fw = 90%. However, the intensity decreased from 90 to 99% of water; this might be caused by the different morphology of the aggregates. In the mixture with a relatively lower water fraction of 90%, molecules may slowly assemble in an ordered fashion which was more emissive, like crystalline clusters, while the solvent mixture with higher ratio of water made it less emissive, because the molecules may agglomerate to form random or amorphous aggregates.16 This was evidenced by FE-SEM analysis of 3a in a 90 and 99% water/ACN mixture.† Inspection of the crystal structure of the compound 3a reveals that the molecules twisted themselves into a propeller-like conformation.† Due to the steric hindrance of the R2 and R3 groups present at adjacent positions, the molecule 3a adopts tetrahydroisoquinoline and methanofluoren-9-ylidene rings in a non-planar conformation around its C–C double bond. This non-planarity allows active intramolecular vibrations and rotations, rendering the molecule non-emissive in the solution state. This structural rigidification effect thus makes the compound a stronger emitter in the condensed phase. Furthermore, Hirshfeld surface analyses of 3a shows that the optimized geometry of 3a was mostly stabilized by intermolecular H⋯H weak contacts (75.4%).
On the basis of the results obtained, all the compounds 3a–p were found to be virtually nonfluorescent in acetonitrile solution, but highly luminescent in the condensed phase (Table 3). The longest absorption maxima for all the compounds (3a–p) were found between 328 and 371 nm in a 90% H2O/CH3CN mixture and between 319 and 357 nm in thin film. The PL spectra of all the compounds (3a–p) showed emission maxima in the range 408–460 nm and 405–452 nm when excited at 320 nm (because irradiating at the λmax, we could not cover all the expected bands) to cover the complete area of the emission intensity from 350–600 nm for all the compounds in solution and in the thin film, respectively. These results demonstrate that norbornene incorporated tetra-substituted alkenes (3a–p) are unique and promising candidates of novel AIE based solid fluorophores.
Table 3 Photophysical properties of synthesized compounds (3a–p)
| Compounds (3a–p) |
λmax,ab (nm) |
λmax,em (nm) |
| Solutiona |
Thin film |
Solutionb |
Thin film |
In CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) mixture at 10 μM. Excitation wavelength: 320 nm. 90) mixture at 10 μM. Excitation wavelength: 320 nm. |
| 3a |
352 |
346 |
423 |
427 |
| 3b |
346 |
336 |
420 |
419 |
| 3c |
335 |
339 |
425 |
426 |
| 3d |
356 |
343 |
423 |
425 |
| 3e |
358 |
350 |
446 |
435 |
| 3f |
343 |
339 |
408 |
405 |
| 3g |
353 |
347 |
426 |
430 |
| 3h |
351 |
332 |
419 |
426 |
| 3i |
333 |
319 |
427 |
418 |
| 3j |
333 |
319 |
435 |
432 |
| 3k |
347 |
332 |
422 |
426 |
| 3l |
354 |
349 |
425 |
425 |
| 3m |
351 |
337 |
420 |
425 |
| 3n |
342 |
347 |
437 |
443 |
| 3o |
371 |
357 |
460 |
452 |
| 3p |
328 |
352 |
421 |
425 |
Conclusions
In conclusion, we have developed a palladium-catalyzed norbornene-mediated domino reaction to access highly complex tetra-substituted olefinic compounds. In this process, three C–C bonds are constructed in a single transformation including one C–H activation process. The synthesized molecules form a new class of AIE system with novel structures and unique properties. Our ongoing research is directed towards development of new methodology to synthesize norbornene functionalized compounds which is currently underway.
Experimental
Reagents and solvents were purchased from commercial sources (Aldrich and Merck). Reagents and solvents (anhydrous DMF-99.8%) were used without further purification unless otherwise noted. Column chromatography was performed on silica gel (100–200 mesh, SRL India). Analytical TLC was performed on precoated aluminium sheets of silica gel 60F254 of 0.2 mm thickness (Merck, Germany). Melting points were determined in capillary tubes and are uncorrected. 1H NMR (400 MHz) and 13C (100 MHz) spectra were recorded in CDCl3 solution with TMS as an internal standard on a Bruker Avance III HD spectrometer. High resolution mass spectra (HRMS-ESI) were recorded using a Thermo Scientific Exactive Orbitrap mass spectrometer. UV-visible absorption spectra were measured using a Shimadzu UV-1800 spectrophotometer. The steady state fluorescence measurements were measured using a Varian Cary Eclipse fluorescence spectrophotometer.
General procedure for the synthesis of 2-bromo-N-benzylpropargylamines (1a–p)
A mixture of CuI (15 mol%), amine S1 (0.50 mmol), aldehyde S2 (0.55 mmol) and alkyne S3 (0.75 mmol) in anhydrous toluene (3 mL) was heated at 100 °C for 3 h. Then the reaction mixture was filtered through Celite and washed with ethyl acetate. After removal of the solvent, the residue was purified by column chromatography on silica gel using petroleum ether/ethyl acetate as the eluent, affording 2-bromo-N-benzylpropargylamine compounds (1a–p).
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-3-phenyl-1-(p-tolyl)prop-2-yn-1-amine (1a). Yield: 86%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.64–7.62 (m, 2H), 7.58 (d, J = 7.5 Hz, 2H), 7.42–7.37 (m, 5H), 7.30 (t, J = 7.2 Hz, 2H), 7.25–7.21 (m, 1H), 7.16–7.13 (m, 3H), 6.95 (s, 1H), 4.91 (s, 1H), 3.92–3.88 (m, 4H), 3.83 (s, 3H), 3.79 (d, J = 13.5 Hz, 1H), 3.63 (d, J = 5.6 Hz, 1H), 3.59 (d, J = 6.2 Hz, 1H), 2.33 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.5, 148.5, 139.6, 137.3, 136.1, 132.1, 130.7, 129.1, 129.0, 128.5, 128.4, 128.4, 128.3, 127.2, 123.5, 115.3, 114.4, 113.4, 88.6, 85.2, 56.3, 56.3, 56.1, 55.0, 53.6, 21.2 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1,3-diphenylprop-2-yn-1-amine (1b). Yield: 84%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 7.6 Hz, 2H), 7.64–7.62 (m, 2H), 7.42–7.37 (m, 5H), 7.34 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H), 7.27–7.23 (m, 2H), 7.10 (s, 1H), 6.95 (s, 1H), 4.94 (s, 1H), 3.91 (d, J = 14.1 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 3.79 (d, J = 13.5 Hz, 1H), 3.63 (d, J = 7.2 Hz, 1H), 3.60 (d, J = 7.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.6, 148.5, 139.5, 139.2, 132.1 (2C), 130.7, 129.1, 128.6, 128.5, 128.4, 128.3, 127.7, 127.3, 123.4, 115.3, 114.5, 113.5, 88.9, 84.9, 56.6, 56.3, 56.1, 55.1, 53.7 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-3-phenyl-1-(o-tolyl)prop-2-yn-1-amine (1c). Yield: 78%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 5.1, 3.9 Hz, 1H), 7.65–7.63 (m, 2H), 7.40–7.38 (m, 3H), 7.28–7.25 (m, 5H), 7.18–7.15 (m, 2H), 7.10 (dd, J = 5.4, 3.5 Hz, 1H), 6.93 (s, 1H), 6.80 (s, 1H), 5.03 (s, 1H), 3.83 (s, 3H), 3.82 (d, J = 3.0 Hz, 1H), 3.79 (d, J = 3.5 Hz, 1H), 3.77 (s, 3H), 3.70 (d, J = 13.6 Hz, 1H), 3.59 (d, J = 12.8 Hz, 1H), 2.10 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 148.4, 148.1, 138.7, 137.5, 136.3, 131.9, 130.6, 130.5, 129.9, 129.8, 128.4, 128.2, 128.0, 127.9, 127.2, 125.4, 123.4, 115.0, 114.6, 114.0, 88.9, 85.4, 56.1, 55.7, 55.6, 54.8, 53.7, 19.0 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1-(4-chlorophenyl)-3-phenylprop-2-yn-1-amine (1d). Yield: 74%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 6.9 Hz, 4H), 7.39–7.38 (m, 5H), 7.32–7.30 (m, 4H), 7.25–7.22 (m, 1H), 7.05 (s, 1H), 6.96 (s, 1H), 4.88 (s, 1H), 3.90–3.86 (m, 4H), 3.84 (s, 3H), 3.76 (d, J = 13.5 Hz, 1H), 3.59 (d, J = 14.2 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.7, 148.5, 139.2, 137.8, 133.5, 132.1 (2C), 130.3, 129.9, 129.1, 128.6, 128.5, 128.4, 127.4, 123.1, 115.4, 114.6, 113.5, 89.2, 84.3, 56.3, 56.1, 56.0, 55.1, 53.8 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1-(naphthalen-1-yl)-3-phenylprop-2-yn-1-amine (1e). Yield: 72%, colourless solid. 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 7.0 Hz, 1H), 7.87–7.69 (m, 5H), 7.43–7.26 (m, 11H), 6.87 (s, 1H), 6.50 (s, 1H), 5.61 (s, 1H), 3.88 (d, J = 14.1 Hz, 1H), 3.81 (d, J = 11.7 Hz, 1H), 3.77 (s, 3H), 3.64 (d, J = 13.9 Hz, 1H), 3.38 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.3, 148.3, 138.6, 134.1, 132.1, 131.6, 130.8, 130.3, 129.0, 128.6 (2C), 128.5, 128.4, 128.2, 127.8, 127.6, 125.5, 125.4, 125.2, 125.0, 123.4, 114.8, 114.1, 113.9, 89.5, 85.2, 56.3, 56.2, 55.5, 54.8, 53.4 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1-cyclohexyl-3-phenylprop-2-yn-1-amine (1f). Yield: 78%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.3 Hz, 2H), 7.42 (d, J = 7.5 Hz, 2H), 7.35–7.29 (m, 5H), 7.24 (d, J = 4.6 Hz, 1H), 7.21 (s, 1H), 6.98 (s, 1H), 3.90–3.85 (m, 8H), 3.67 (d, J = 14.6 Hz, 1H), 3.57 (d, J = 13.8 Hz, 1H), 3.26 (d, J = 10.3 Hz, 1H), 2.31 (d, J = 12.6 Hz, 1H), 2.09 (d, J = 12.7 Hz, 1H), 1.71–1.62 (m, 4H), 1.27–1.08 (m, 3H), 0.95–0.77 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.5, 148.4, 139.8, 132.0, 130.9, 129.0, 128.4, 128.4, 128.0, 127.1, 123.8, 115.4, 114.3, 113.2, 87.2, 86.5, 59.0, 56.3, 56.1, 55.5, 54.4, 40.0, 31.5, 30.9, 26.7, 26.3, 26.1 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-3-phenyl-1-(thiophen-2-yl)prop-2-yn-1-amine (1g). Yield: 80%, brown liquid. 1H NMR (400 MHz, CDCl3) δ 7.62–7.60 (m, 2H), 7.49 (d, J = 7.5 Hz, 2H), 7.38–7.36 (m, 4H), 7.32 (t, J = 7.3 Hz, 3H), 7.27–7.22 (m, 2H), 6.95–6.94 (m, 2H), 5.06 (s, 1H), 3.99–3.95 (m, 4H), 3.90 (d, J = 13.7 Hz, 1H), 3.84 (s, 3H), 3.67 (d, J = 14.7 Hz, 1H), 3.63 (d, J = 13.8 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.7, 148.5, 144.6, 139.2, 132.1, 130.3, 128.8, 128.6, 128.5, 128.5, 127.3, 126.5, 126.5, 125.6, 123.0, 115.2, 114.2, 112.8, 87.4, 84.3, 56.3, 56.2, 54.8, 53.6, 53.2 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1-(furan-3-yl)-3-phenylprop-2-yn-1-amine (1h). Yield: 86%, yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.60–7.56 (m, 3H), 7.42–7.36 (m, 6H), 7.31 (t, J = 7.3 Hz, 2H), 7.25–7.21 (m, 1H), 7.15 (s, 1H), 6.96 (s, 1H), 6.53 (s, 1H), 4.78 (s, 1H), 3.90–3.84 (m, 8H), 3.68 (d, J = 14.3 Hz, 1H), 3.61 (d, J = 13.7 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.6 (2C), 143.5, 141.7, 139.5, 132.1 (2C), 130.6, 128.9, 128.5, 128.5, 127.3, 125.1, 123.2, 115.4, 114.4, 113.1, 110.3, 86.3, 85.1, 56.3, 56.1, 54.8, 53.6, 49.3 ppm.
N-Benzyl-N-(2-bromobenzyl)-1-(4-methoxyphenyl)-3-phenylprop-2-yn-1-amine (1i). Yield: 80%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.66–7.62 (m, 5H), 7.49 (d, J = 7.9 Hz, 1H), 7.43–7.37 (m, 5H), 7.32–7.27 (m, 3H), 7.22 (t, J = 7.3 Hz, 1H), 7.06 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 8.2 Hz, 2H), 4.89 (s, 1H), 3.97 (d, J = 14.4 Hz, 1H), 3.80–3.78 (m, 4H), 3.69 (d, J = 14.4 Hz, 1H), 3.60 (d, J = 13.5 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 159.2, 139.4, 138.7, 132.8, 132.1, 131.1, 130.9, 129.6, 129.2, 128.5, 128.5, 128.4, 128.4, 127.5, 127.2, 124.8, 123.5, 113.6, 88.8, 85.2, 56.0, 55.4, 55.0, 54.0 ppm.
N-Benzyl-N-(2-bromobenzyl)-1-(furan-3-yl)-3-phenylprop-2-yn-1-amine (1j). Yield: 78%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 7.6 Hz, 1H), 7.60–7.56 (m, 3H), 7.50 (d, J = 7.9 Hz, 1H), 7.43 (d, J = 7.5 Hz, 2H), 7.38–7.35 (m, 4H), 7.32–7.27 (m, 3H), 7.23 (t, J = 7.2 Hz, 1H), 7.07 (t, J = 7.6 Hz, 1H), 6.57 (s, 1H), 4.78 (s, 1H), 3.96 (d, J = 14.7 Hz, 1H), 3.86 (d, J = 13.7 Hz, 1H), 3.76 (d, J = 14.7 Hz, 1H), 3.61 (d, J = 13.7 Hz, 1H) ppm; 13C NMR (101 MHz, CDCl3) δ 143.5 (2C), 141.7, 139.3, 138.6, 132.9, 132.1 (2C), 130.5, 129.0, 128.5, 128.5, 127.5, 127.2, 125.0, 124.7, 123.2, 110.4, 86.4, 85.0, 54.9, 54.0, 49.3 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1,3-di-p-tolylprop-2-yn-1-amine (1k). Yield: 86%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 7.7 Hz, 2H), 7.51 (d, J = 7.7 Hz, 2H), 7.40 (d, J = 7.5 Hz, 2H), 7.30 (t, J = 7.3 Hz, 2H), 7.23 (d, J = 8.8 Hz, 1H), 7.19 (d, J = 7.8 Hz, 2H), 7.15–7.12 (m, 3H), 6.94 (s, 1H), 4.89 (s, 1H), 3.90–3.87 (d, J = 15.1 Hz, 4H), 3.83 (s, 3H), 3.77 (d, J = 13.5 Hz, 1H), 3.62 (d, J = 7.2 Hz, 1H), 3.58 (d, J = 7.8 Hz, 1H), 2.39 (s, 3H), 2.33 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.5, 148.5, 139.6, 138.4, 137.3, 136.3, 132.0, 130.8, 129.3, 129.1, 128.9, 128.4, 128.4, 127.2, 120.4, 115.3, 114.5, 113.5, 88.7, 84.4, 56.3, 56.3, 56.1, 55.0, 53.7, 21.6, 21.2 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-1-(thiophen-2-yl)-3-(p-tolyl)prop-2-yn-1-amine (1l). Yield: 82%, brown liquid. 1H NMR (400 MHz, CDCl3) δ 7.51–7.47 (m, 4H), 7.36 (s, 1H), 7.31 (t, J = 7.3 Hz, 3H), 7.26–7.23 (m, 2H), 7.19 (d, J = 7.8 Hz, 2H), 6.95–6.93 (m, 2H), 5.05 (s, 1H), 3.98–3.95 (m, 4H), 3.89 (d, J = 14.2 Hz, 1H), 3.83 (s, 3H), 3.68–3.60 (m, 2H), 2.39 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.7, 148.5, 144.8, 139.2, 138.7, 132.0, 130.4, 129.3, 128.9, 128.5, 127.3, 126.5, 126.5, 125.6, 120.0, 115.2, 114.2, 112.8, 87.5, 83.6, 56.3, 56.2, 54.8, 53.6, 53.2, 21.7 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-3-(4-methoxyphenyl)-1-(p-tolyl)prop-2-yn-1-amine (1m). Yield: 80%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.57 (t, J = 8.4 Hz, 4H), 7.40 (d, J = 7.6 Hz, 2H), 7.30 (t, J = 7.4 Hz, 2H), 7.22 (t, J = 7.3 Hz, 1H), 7.14 (d, J = 10.2 Hz, 3H), 6.94 (s, 1H), 6.91 (d, J = 8.5 Hz, 2H), 4.88 (s, 1H), 3.90–3.87 (m, 4H), 3.85 (s, 3H), 3.83 (s, 3H), 3.77 (d, J = 13.7 Hz, 1H), 3.61 (d, J = 8.4 Hz, 1H), 3.58 (d, J = 9.1 Hz, 1H), 2.33 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 159.7, 148.5, 148.5, 139.7, 137.3, 136.4, 133.5, 130.9, 129.1, 128.9, 128.4, 128.4, 127.2, 115.6, 115.3, 114.4, 114.2, 113.4, 88.4, 83.3, 56.3, 56.3, 56.1, 55.5, 55.0, 53.7, 21.2 ppm.
10,13-Dimethyl-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl(4-(3-(benzyl(2-bromo-4,5-dimethoxybenzyl)amino)-3-(p-tolyl)prop-1-yn-1-yl)phenyl)carbamate (1n). Yield: 65%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.57–7.54 (m, 4H), 7.40 (d, J = 7.5 Hz, 4H), 7.30 (t, J = 7.3 Hz, 2H), 7.24–7.20 (m, 1H), 7.15 (d, J = 7.7 Hz, 2H), 7.12 (s, 1H), 6.94 (s, 1H), 6.64 (s, 1H), 5.42 (d, J = 2.7 Hz, 1H), 4.88 (s, 1H), 4.66–4.60 (m, 1H), 3.90–3.87 (m, 4H), 3.83 (s, 3H), 3.77 (d, J = 13.5 Hz, 1H), 3.61 (d, J = 5.0 Hz, 1H), 3.57 (d, J = 5.8 Hz, 1H), 2.45–2.42 (m, 1H), 2.38 (d, J = 12.2 Hz, 1H), 2.33 (s, 3H), 2.00 (t, J = 14.2 Hz, 3H), 1.91–1.83 (m, 2H), 1.68–1.59 (m, 2H), 1.54–1.44 (m, 5H), 1.38–1.25 (m, 4H), 1.20–1.08 (m, 7H), 1.04–1.01 (m, 6H), 0.92 (d, J = 6.3 Hz, 3H), 0.87 (d, J = 6.5 Hz, 6H), 0.69 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 152.9, 148.5, 148.5, 139.7, 139.6, 138.2, 137.3, 136.2, 132.9 (2), 130.8, 129.1, 129.0, 128.4, 127.2, 123.0, 118.3, 118.1, 115.3, 114.5, 113.4, 88.4, 84.4, 56.8, 56.3, 56.3, 56.3, 56.1, 55.0, 53.7, 50.2, 42.5, 39.9, 39.7, 38.6, 37.1, 36.7, 36.3, 36.0, 32.1, 32.0, 28.4, 28.2, 28.2, 24.4, 23.98, 23.0, 22.7, 21.3, 21.2, 19.5, 18.9, 12.0 ppm.
N-Benzyl-N-(2-bromo-4,5-dimethoxybenzyl)-3-(thiophen-2-yl)-1-(p-tolyl)prop-2-yn-1-amine (1o). Yield: 78%, brown liquid. 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.0 Hz, 2H), 7.40 (d, J = 7.2 Hz, 2H), 7.35 (dd, J = 3.6, 1.0 Hz, 1H), 7.32–7.28 (m, 3H), 7.23 (t, J = 7.3 Hz, 1H), 7.15 (d, J = 7.9 Hz, 2H), 7.10 (s, 1H), 7.04 (dd, J = 5.2, 3.6 Hz, 1H), 6.95 (s, 1H), 4.91 (s, 1H), 3.88–3.83 (m, 7H), 3.77 (d, J = 13.5 Hz, 1H), 3.61 (d, J = 9.2 Hz, 1H), 3.57 (d, J = 8.7 Hz, 1H), 2.33 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.6, 148.5, 139.5, 137.4, 135.8, 132.1, 130.6, 129.1, 129.0, 128.4, 128.4, 127.3, 127.1, 127.0, 123.4, 115.3, 114.5, 113.4, 89.5, 81.7, 56.6, 56.3, 56.1, 55.0, 53.7, 21.2 ppm.
N-(2-Bromo-4,5-dimethoxybenzyl)-N-methyl-3-phenyl-1-(p-tolyl)prop-2-yn-1-amine (1p). Yield: 82%, colourless liquid. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 7.1 Hz, 4H), 7.35 (d, J = 4.9 Hz, 3H), 7.16 (d, J = 7.6 Hz, 2H), 7.07 (s, 1H), 7.01 (s, 1H), 4.92 (s, 1H), 3.88 (s, 3H), 3.85–3.83 (m, 4H), 3.64 (d, J = 13.7 Hz, 1H), 2.35 (s, 3H), 2.25 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.6, 148.4, 137.3, 136.1, 132.0, 130.5, 129.0, 128.5, 128.4, 128.3, 123.4, 115.6, 114.7, 113.6, 88.6, 85.1, 59.7, 58.2, 56.3, 56.2, 37.8, 21.2 ppm.
General procedure for the synthesis of tetra substituted olefins (3a–p)
A solution of 2-bromo-N-benzylpropargylamines 1a–p (0.10 mmol) in anhydrous DMF (3 mL) was added to a mixture of Pd(PPh3)4 (10 mol%), norbornene 2 (2.0 equiv.) and K2CO3 (3.0 equiv.) in a clean, dry, two-necked round-bottomed flask under N2. The reaction mixture was stirred at 100 °C for 3 h. Afterwards, the reaction mixture was cooled to room temperature and ethyl acetate and water were added. The organic layer was separated, dried with anhydrous Na2SO4, and concentrated under reduced pressure. The crude material was purified by column chromatography (silica gel, petroleum ether/ethyl acetate) to afford 3a–p.
(Z)-2-Benzyl-6,7-dimethoxy-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline (3a). Yield: 64%, white solid, mp 198–202 °C. 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.42 (d, J = 7.7 Hz, 2H), 7.19 (d, J = 7.4 Hz, 1H), 7.12–7.03 (m, 8H), 6.78 (d, J = 3.6 Hz, 2H), 6.37 (s, 1H), 5.25 (s, 1H), 3.99 (s, 3H), 3.94 (d, J = 17.3 Hz, 1H), 3.80 (s, 3H), 3.67 (d, J = 13.3 Hz, 1H), 3.52–3.48 (m, 2H), 3.39 (d, J = 6.0 Hz, 1H), 3.19 (d, J = 6.1 Hz, 1H), 2.74 (s, 1H), 2.39 (d, J = 3.0 Hz, 1H), 2.32 (s, 3H), 1.78–1.70 (m, 1H), 1.65–1.61 (m, 2H), 1.53–1.46 (m, 1H), 1.01 (d, J = 10.2 Hz, 1H), 0.92 (d, J = 10.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.4, 148.2, 146.9, 144.6, 143.5, 139.4, 138.3, 136.5, 129.1, 128.7, 128.4, 128.1, 128.0, 127.3, 127.2, 127.1, 126.6, 126.2, 124.2, 124.0, 112.1, 109.1, 62.1, 58.6, 56.1, 55.8, 53.7, 52.2, 49.9, 45.0, 42.1, 33.6, 30.9, 27.4, 21.3 ppm; HRMS (ESI): calcd for C39H40NO2 [M + H]+ 554.3059; found 554.3064.
(Z)-2-Benzyl-6,7-dimethoxy-3-phenyl-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3b). Yield: 64%, white solid, mp 230–234 °C. 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.6 Hz, 2H), 7.48 (s, 1H), 7.30 (t, J = 7.4 Hz, 2H), 7.24–7.18 (m, 2H), 7.12–7.04 (m, 6H), 6.80–6.74 (m, 2H), 6.37 (s, 1H), 5.28 (s, 1H), 3.99 (s, 3H), 3.95 (d, J = 17.3 Hz, 1H), 3.80 (s, 3H), 3.69 (d, J = 13.2 Hz, 1H), 3.52 (d, J = 14.5 Hz, 2H), 3.39 (d, J = 6.0 Hz, 1H), 3.21 (d, J = 6.1 Hz, 1H), 2.74 (s, 1H), 2.39 (d, J = 2.5 Hz, 1H), 1.78–1.70 (m, 1H), 1.65–1.61 (m, 2H), 1.52–1.46 (m, 1H), 1.01 (d, J = 10.2 Hz, 1H), 0.93 (d, J = 10.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.4, 148.2, 146.9, 144.8, 143.4, 141.4, 139.3, 128.7, 128.5, 128.4, 128.2, 128.0, 127.3, 127.1, 127.0, 126.8, 126.7, 126.2, 124.1, 124.0, 112.1, 109.1, 62.3, 58.7, 56.1, 55.8, 53.7, 52.2, 49.7, 45.0, 42.1, 33.6, 30.9, 27.3 ppm; HRMS (ESI): calcd for C38H38NO2 [M + H]+ 540.2903; found 540.2885.
(Z)-2-Benzyl-6,7-dimethoxy-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(o-tolyl)-1,2,3,4-tetrahydroisoquinoline (3c). Yield: 62%, white solid, mp 188–192 °C. 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 1H), 7.23 (d, J = 7.3 Hz, 2H), 7.17–7.02 (m, 8H), 6.94 (dd, J = 17.9, 7.2 Hz, 2H), 6.72 (d, J = 7.7 Hz, 1H), 6.41 (s, 1H), 5.28 (s, 1H), 4.04 (s, 3H), 3.84 (s, 3H), 3.69 (d, J = 17.1 Hz, 1H), 3.58 (d, J = 13.1 Hz, 1H), 3.48 (d, J = 13.1 Hz, 1H), 3.41 (dd, J = 11.7, 5.5 Hz, 2H), 3.28 (d, J = 6.2 Hz, 1H), 2.74 (s, 1H), 2.40 (s, 3H), 2.39 (d, J = 4.2 Hz, 1H), 1.78–1.70 (m, 1H), 1.67–1.63 (m, 2H), 1.54–1.48 (m, 1H), 0.99 (d, J = 11.3 Hz, 1H), 0.88 (d, J = 10.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 148.5, 148.4, 147.2, 144.3, 143.9, 139.4, 139.1, 138.4, 131.1, 129.4, 128.5, 128.2, 127.9, 127.4, 127.1, 127.0, 126.8, 126.1, 125.4, 124.5, 124.1, 111.8, 109.5, 62.4, 58.3, 56.2, 55.9, 53.8, 52.2, 49.3, 44.5, 42.2, 33.49, 30.9, 27.4, 19.6 ppm.
(Z)-2-Benzyl-3-(4-chlorophenyl)-6,7-dimethoxy-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3d). Yield: 56%, white solid, mp 190–194 °C. 1H NMR (400 MHz, CDCl3) δ 7.49 (s, 1H), 7.47 (d, J = 4.2 Hz, 2H), 7.26 (d, J = 7.5 Hz, 3H), 7.20 (d, J = 7.4 Hz, 1H), 7.13 (t, J = 7.3 Hz, 1H), 7.04 (s, 4H), 6.79 (t, J = 7.5 Hz, 1H), 6.70 (d, J = 7.7 Hz, 1H), 6.37 (s, 1H), 5.22 (s, 1H), 3.99 (s, 3H), 3.91 (d, J = 17.0 Hz, 1H), 3.81 (s, 3H), 3.67 (d, J = 13.2 Hz, 1H), 3.51 (dd, J = 15.2, 10.5 Hz, 2H), 3.40 (d, J = 6.0 Hz, 1H), 3.20 (d, J = 6.1 Hz, 1H), 2.73 (s, 1H), 2.40 (d, J = 3.1 Hz, 1H), 1.78–1.71 (m, 1H), 1.65–1.62 (m, 2H), 1.53–1.46 (m, 1H), 1.02 (d, J = 10.2 Hz, 1H), 0.92 (d, J = 10.2 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.6, 148.3, 147.0, 145.2 (2), 143.3, 140.0, 139.1, 132.7, 130.0, 128.7, 128.6, 128.3, 128.0, 127.1, 126.8, 126.2, 126.2, 124.2, 124.0, 112.0, 109.0, 61.7, 58.6, 56.1, 55.8, 53.7, 52.2, 49.6, 45.0, 42.1, 33.6, 30.9, 27.3 ppm; HRMS (ESI): calcd for C38H37ClNO2 [M + H]+ 574.2513; found 574.2514.
(Z)-2-Benzyl-6,7-dimethoxy-3-(naphthalen-1-yl)-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3e). Yield: 58%, white solid, mp 232–236 °C. 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 8.4 Hz, 1H), 7.86 (d, J = 8.1 Hz, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.64 (s, 1H), 7.49 (t, J = 7.4 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.39 (d, J = 7.1 Hz, 1H), 7.23 (d, J = 7.9 Hz, 2H), 7.14–7.09 (m, 6H), 6.71 (t, J = 7.5 Hz, 1H), 6.60 (d, J = 7.7 Hz, 1H), 6.40 (s, 1H), 5.88 (s, 1H), 4.07 (s, 3H), 3.83 (s, 3H), 3.71–3.63 (m, 3H), 3.45–3.41 (m, 2H), 3.34 (d, J = 6.2 Hz, 1H), 2.79 (s, 1H), 2.40 (d, J = 2.7 Hz, 1H), 1.79–1.70 (m, 3H), 1.53–1.50 (m, 1H), 1.01 (d, J = 10.2 Hz, 1H), 0.90 (d, J = 10.6 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.5, 148.3, 147.2, 144.8, 143.6, 139.0, 136.7, 134.5, 132.1, 129.4, 128.7, 128.4, 128.2, 128.0, 127.8, 127.0, 126.9, 126.7, 126.3, 126.2, 125.7, 125.6, 125.4, 125.1, 124.9, 124.1, 111.8, 109.5, 61.2, 58.5, 56.3, 55.9, 53.8, 52.2, 49.8, 44.9, 42.2, 33.5, 30.9, 27.4 ppm; HRMS (ESI): calcd for C42H40NO2 [M + H]+ 590.3059; found 590.3065.
(Z)-2-Benzyl-3-cyclohexyl-6,7-dimethoxy-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3f). Yield: 74%, white solid, mp 214–218 °C. 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 7.8 Hz, 1H), 7.18 (d, J = 10.9 Hz, 2H), 7.16–7.11 (m, 1H), 7.07 (d, J = 3.0 Hz, 3H), 6.96 (d, J = 5.0 Hz, 3H), 6.47 (s, 1H), 4.14 (d, J = 17.9 Hz, 1H), 4.03 (d, J = 10.4 Hz, 1H), 3.95 (s, 3H), 3.86 (s, 3H), 3.46 (d, J = 17.9 Hz, 1H), 3.38 (d, J = 14.0 Hz, 1H), 3.25 (d, J = 6.3 Hz, 1H), 3.17 (d, J = 13.9 Hz, 1H), 2.90 (d, J = 6.4 Hz, 1H), 2.55 (s, 1H), 2.39 (d, J = 11.9 Hz, 1H), 2.33 (d, J = 3.2 Hz, 1H), 1.82 (d, J = 4.9 Hz, 1H), 1.77 (d, J = 12.8 Hz, 1H), 1.71–1.66 (m, 3H), 1.55–1.48 (m, 2H), 1.43–1.31 (m, 2H), 1.27–1.14 (m, 3H), 1.09 (dd, J = 21.5, 10.7 Hz, 2H), 0.95 (dd, J = 20.9, 10.1 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.4, 148.2, 146.5, 143.2, 143.0, 140.3, 129.7, 128.4, 128.1, 127.9, 127.9, 127.7, 126.4, 126.0, 124.4, 124.3, 112.4, 108.5, 63.6, 59.5, 55.9, 55.9, 53.2, 51.9, 49.2, 46.0, 42.0, 38.6, 33.6, 31.1, 31.1, 30.6, 27.3, 27.2, 26.9, 26.8 ppm; HRMS (ESI): calcd for C38H44NO2 [M + H]+ 546.3372; found 546.3358.
(Z)-2-Benzyl-6,7-dimethoxy-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(thiophen-2-yl)-1,2,3,4-tetrahydroisoquinoline (3g). Yield: 60%, white solid, mp 206–210 °C. 1H NMR (400 MHz, CDCl3) δ 7.43 (s, 1H), 7.26–7.23 (m, 1H), 7.19 (d, J = 7.4 Hz, 1H), 7.14 (d, J = 7.2 Hz, 1H), 7.10–7.04 (m, 5H), 6.93 (d, J = 7.7 Hz, 1H), 6.90 (d, J = 3.0 Hz, 2H), 6.85 (t, J = 7.4 Hz, 1H), 6.44 (s, 1H), 5.48 (s, 1H), 4.08 (d, J = 17.1 Hz, 1H), 3.98 (s, 3H), 3.83 (s, 3H), 3.68 (d, J = 13.3 Hz, 1H), 3.60 (d, J = 17.1 Hz, 1H), 3.54 (d, J = 13.3 Hz, 1H), 3.37 (d, J = 6.1 Hz, 1H), 3.15 (d, J = 6.2 Hz, 1H), 2.68 (s, 1H), 2.37 (d, J = 3.2 Hz, 1H), 1.76–1.69 (m, 1H), 1.63–1.59 (m, 2H), 1.51–1.45 (m, 1H), 0.99 (d, J = 10.2 Hz, 1H), 0.89 (d, J = 9.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.5, 148.2, 147.0, 145.5, 143.9, 143.1, 138.8, 128.6, 128.3, 128.1, 127.4, 127.3, 126.8, 126.6, 126.5, 126.4, 126.0, 125.2, 124.2, 124.1, 112.2, 109.1, 59.1, 58.6, 56.1, 55.9, 53.6, 52.2, 50.0, 44.8, 42.1, 33.5, 30.8, 27.4 ppm; HRMS (ESI): calcd for C36H36NO2S [M + H]+ 546.2467; found 546.2473.
(Z)-2-Benzyl-3-(furan-3-yl)-6,7-dimethoxy-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3h). Yield: 57%, white solid, mp 204–208 °C. 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 1H), 7.37 (s, 1H), 7.19 (d, J = 5.3 Hz, 2H), 7.17–7.07 (m, 6H), 6.98 (d, J = 7.7 Hz, 1H), 6.89 (t, J = 7.4 Hz, 1H), 6.44 (d, J = 10.8 Hz, 2H), 5.25 (s, 1H), 3.97 (s, 3H), 3.90 (d, J = 16.8 Hz, 1H), 3.84 (s, 3H), 3.60 (dd, J = 18.8, 15.2 Hz, 2H), 3.50 (d, J = 13.2 Hz, 1H), 3.36 (d, J = 6.1 Hz, 1H), 3.12 (d, J = 6.2 Hz, 1H), 2.65 (s, 1H), 2.36 (d, J = 3.2 Hz, 1H), 1.76–1.68 (m, 1H), 1.62–1.59 (d, J = 10.4 Hz, 2H), 1.50–1.42 (m, 1H), 0.98 (d, J = 10.2 Hz, 1H), 0.88 (d, J = 10.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.4, 148.2, 147.0, 143.4, 142.9, 142.8, 141.6, 138.9, 128.7, 128.1, 128.1 (2), 127.7, 126.9, 126.8, 126.2, 124.5, 124.4, 124.1, 112.1, 111.1, 109.1, 58.4, 56.1, 55.9, 55.1, 53.6, 52.2, 50.2, 44.7, 42.1, 33.5, 30.8, 27.4 ppm; HRMS (ESI): calcd for C36H36NO3 [M + H]+ 530.2695; found 530.2677.
(Z)-2-Benzyl-3-(4-methoxyphenyl)-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3i). Yield: 63%, white foam, mp 84–88 °C. 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 8.3 Hz, 2H), 7.29 (t, J = 7.7 Hz, 1H), 7.20–7.13 (m, 3H), 7.08–7.04 (m, 5H), 6.90 (d, J = 7.4 Hz, 1H), 6.84–6.82 (m, 4H), 5.26 (s, 1H), 3.99 (d, J = 17.5 Hz, 1H), 3.76 (s, 3H), 3.66 (d, J = 13.2 Hz, 1H), 3.57 (d, J = 17.3 Hz, 1H), 3.49 (d, J = 13.3 Hz, 1H), 3.35 (d, J = 6.1 Hz, 1H), 3.16 (d, J = 6.1 Hz, 1H), 2.66 (s, 1H), 2.39 (d, J = 2.9 Hz, 1H), 1.77–1.70 (m, 1H), 1.64–1.60 (m, 2H), 1.48–1.41 (m, 1H), 0.99 (d, J = 9.8 Hz, 1H), 0.87 (d, J = 9.9 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 158.7, 148.5, 146.6, 143.3, 139.4, 134.8, 134.8, 133.3 (2), 129.6, 129.0, 128.8, 128.4, 128.0, 126.9, 126.8, 126.7, 126.2, 125.7, 124.5, 124.1, 113.8, 61.8, 58.5, 55.3, 53.6, 52.0, 49.8, 44.9, 41.9, 33.5, 30.1, 27.1 ppm; HRMS (ESI): calcd for C37H36NO [M + H]+ 510.2797; found 510.2788.
(Z)-2-Benzyl-3-(furan-3-yl)-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-1,2,3,4-tetrahydroisoquinoline (3j). Yield: 53%, white foam, mp 72–76 °C. 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1H), 7.37 (s, 1H), 7.29 (t, J = 7.5 Hz, 1H), 7.20 (d, J = 6.2 Hz, 3H), 7.18–7.06 (m, 6H), 6.99 (dd, J = 11.9, 7.9 Hz, 2H), 6.90 (t, J = 7.3 Hz, 1H), 6.46 (s, 1H), 5.28 (s, 1H), 3.99 (d, J = 17.0 Hz, 1H), 3.65–3.61 (m, 2H), 3.51 (d, J = 13.3 Hz, 1H), 3.32 (d, J = 6.0 Hz, 1H), 3.09 (d, J = 6.1 Hz, 1H), 2.58 (s, 1H), 2.36 (s, 1H), 1.76–1.68 (m, J = 16.3, 1H), 1.61–1.58 (m, 2H), 1.46–1.40 (m, 1H), 0.97 (d, J = 10.2 Hz, 1H), 0.83 (d, J = 10.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.5, 145.1, 143.2, 143.0, 141.6, 138.9, 135.0, 134.6, 128.9, 128.8, 128.5, 128.1, 127.5, 126.9, 126.8, 126.8, 126.3, 125.8, 124.7, 124.6, 124.2, 111.0, 58.3, 55.2, 53.5, 51.9, 50.4, 44.7, 41.9, 33.4, 30.0, 27.13 ppm; HRMS (ESI): calcd for C34H32NO [M + H]+ 470.2484; found 470.2468.
(Z)-2-Benzyl-6,7-dimethoxy-4-(6-methyl-2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline (3k). Yield: 58%, white solid, mp 208–212 °C. 1H NMR (400 MHz, CDCl3) δ 7.45 (s, 1H), 7.42 (d, J = 7.7 Hz, 2H), 7.11–7.03 (m, 7H), 6.99 (s, 1H), 6.67 (d, J = 7.9 Hz, 1H), 6.58 (d, J = 7.8 Hz, 1H), 6.36 (s, 1H), 5.25 (s, 1H), 3.98 (s, 3H), 3.93 (d, J = 17.3 Hz, 1H), 3.79 (s, 3H), 3.67 (d, J = 13.3 Hz, 1H), 3.51 (s, 1H), 3.47 (d, J = 4.8 Hz, 1H), 3.33 (d, J = 6.0 Hz, 1H), 3.17 (d, J = 6.1 Hz, 1H), 2.72 (s, 1H), 2.36 (d, J = 2.6 Hz, 1H), 2.31 (s, 3H), 2.29 (s, 3H), 1.76–1.69 (m, 1H), 1.63–1.60 (m, 2H), 1.50–1.42 (m, 1H), 0.98 (dd, J = 21.9, 10.1 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.3, 148.2, 146.9, 144.6, 140.9, 139.5, 138.4, 138.0, 136.5, 129.1, 128.8, 128.5, 127.9, 127.4, 127.2, 127.1, 126.5, 126.1, 124.6, 124.0, 112.1, 109.1, 62.2, 58.6, 56.1, 55.8, 53.6, 52.3, 49.5, 45.0, 42.1, 33.6, 30.9, 27.4, 21.5, 21.3 ppm; HRMS (ESI): calcd for C40H42NO2 [M + H]+ 568.3216; found 568.3200.
(Z)-2-Benzyl-6,7-dimethoxy-4-(6-methyl-2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(thiophen-2-yl)-1,2,3,4-tetrahydroisoquinoline (3l). Yield: 52%, white solid, mp 206–210 °C. 1H NMR (400 MHz, CDCl3) δ 7.42 (s, 1H), 7.24–7.23 (m, 1H), 7.10–7.05 (m, 5H), 6.99 (s, 1H), 6.89 (d, J = 2.2 Hz, 2H), 6.82 (d, J = 7.9 Hz, 1H), 6.65 (d, J = 7.9 Hz, 1H), 6.43 (s, 1H), 5.47 (s, 1H), 4.07 (d, J = 17.1 Hz, 1H), 3.97 (s, 3H), 3.82 (s, 3H), 3.68 (d, J = 13.3 Hz, 1H), 3.58 (d, J = 17.4 Hz, 1H), 3.53 (d, J = 13.5 Hz, 1H), 3.32 (d, J = 6.1 Hz, 1H), 3.13 (d, J = 6.1 Hz, 1H), 2.68 (s, 1H), 2.35 (d, J = 3.3 Hz, 1H), 2.31 (s, 3H), 1.76–1.68 (m, 1H), 1.60–1.58 (m, 2H), 1.50–1.43 (m, 1H), 0.99 (d, J = 10.2 Hz, 1H), 0.92 (d, J = 10.1 Hz, 1H) ppm; 13C NMR (101 MHz, CDCl3) δ 148.4, 148.3, 147.0, 145.7, 144.0, 140.5, 138.9, 138.2, 128.7, 128.1, 127.3, 127.3, 126.8, 126.6, 126.5, 126.3, 125.9, 125.1, 124.7, 124.0, 112.1, 109.1, 59.2, 58.6, 56.1, 55.8, 53.5, 52.3, 50.0, 44.7, 42.1, 33.5, 30.8, 27.4, 21.5 ppm; HRMS (ESI): calcd for C37H38NO2S [M + H]+ 560.2623; found 560.2635.
(Z)-2-Benzyl-6,7-dimethoxy-4-(6-methoxy-2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline (3m). Yield: 57%, white foam, mp 78–82 °C. 1H NMR (400 MHz, CDCl3) δ 7.44–7.40 (m, 3H), 7.11–7.05 (m, 7H), 6.73 (s, 1H), 6.67 (d, J = 8.5 Hz, 1H), 6.37 (s, 1H), 6.33 (d, J = 8.4 Hz, 1H), 5.18 (s, 1H), 3.99 (s, 3H), 3.95 (d, J = 17.9 Hz, 1H), 3.80 (s, 3H), 3.77 (s, 3H), 3.69 (d, J = 13.0 Hz, 1H), 3.53 (d, J = 3.6 Hz, 1H), 3.49 (d, J = 8.5 Hz, 1H), 3.34 (d, J = 5.8 Hz, 1H), 3.19 (d, J = 6.1 Hz, 1H), 2.73 (s, 1H), 2.37 (s, 1H), 2.31 (s, 3H), 1.78–1.69 (m, 1H), 1.65–1.61 (m, 2H), 1.50–1.45 (m, 1H), 1.00 (q, J = 10.0 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 160.1, 150.1, 148.1, 146.9, 144.1, 139.5, 138.5, 136.5, 136.5, 129.1, 128.8, 128.5, 127.9, 127.3, 127.0, 126.6, 125.0, 124.9, 111.9, 111.8, 109.3, 109.1, 62.0, 58.6, 56.1, 55.8, 55.5, 53.7, 52.4, 49.7, 44.9, 42.0, 33.6, 30.8, 27.3, 21.3 ppm; HRMS (ESI): calcd for C40H42NO3 [M + H]+ 584.3165; found 584.3146.
(Z)-10,13-Dimethyl-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl(9-(2-benzyl-6,7-dimethoxy-3-(p-tolyl)-2,3-dihydroisoquinolin-4(1H)-ylidene)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanofluoren-6-yl)carbamate (3n). Yield: 56%, white solid, mp 176–180 °C. 1H NMR (400 MHz, CDCl3) δ 7.44 (s, 1H), 7.41 (d, J = 7.7 Hz, 2H), 7.11–7.06 (m, 8H), 6.67 (d, J = 8.3 Hz, 1H), 6.57 (d, J = 8.0 Hz, 1H), 6.53 (s, 1H), 6.36 (s, 1H), 5.41 (d, J = 1.9 Hz, 1H), 5.18 (s, 1H), 4.63–4.57 (m, 1H), 3.98 (s, 3H), 3.93 (d, J = 17.1 Hz, 1H), 3.79 (s, 3H), 3.68 (d, J = 13.2 Hz, 1H), 3.52 (s, 1H), 3.48 (d, J = 5.2 Hz, 1H), 3.35 (d, J = 5.9 Hz, 1H), 3.18 (d, J = 6.1 Hz, 1H), 2.72 (s, 1H), 2.46–2.35 (m, 3H), 2.31 (s, 3H), 2.03–1.95 (m, 3H), 1.90–1.79 (m, 2H), 1.76–1.62 (m, 4H), 1.54–1.43 (m, 6H), 1.35–1.26 (m, 5H), 1.20–1.09 (m, 7H), 1.03–0.98 (m, 8H), 0.92 (d, J = 6.4 Hz, 3H), 0.87 (d, J = 6.6 Hz, 6H), 0.68 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 153.0, 149.6, 148.2, 146.9, 144.0, 139.7, 139.4, 138.8, 138.3, 138.0, 137.6, 136.5, 129.9, 129.1, 128.8, 128.4, 128.0, 127.2, 127.1, 126.6, 125.9, 124.5, 122.9, 111.9, 109.1, 62.0, 58.6, 56.8, 56.3, 56.1, 55.8, 53.7, 52.4, 50.1, 49.6, 49.6, 45.0, 42.5, 42.0, 39.9, 39.7, 38.6, 37.1, 36.7, 36.3, 35.9, 33.6, 32.1, 32.0, 30.8, 28.4, 28.2, 27.3, 24.4, 24.0, 23.0, 22.7, 21.3, 21.2, 19.5, 18.9, 12.0 ppm; HRMS (ESI): calcd for C67H85N2O4 [M + H]+ 981.6509; found 981.6492.
(Z)-2-Benzyl-6,7-dimethoxy-4-(5,6,7,7a-tetrahydro-3bH-4,7-methanoindeno[2,1-b]thiophen-8(4H)-ylidene)-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline (3o). Yield: 65%, white foam, mp 92–96 °C. 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H), 7.35 (d, J = 7.1 Hz, 2H), 7.30 (d, J = 7.7 Hz, 2H), 7.27–7.20 (m, 3H), 7.10 (d, J = 4.9 Hz, 1H), 7.03 (d, J = 7.6 Hz, 2H), 6.71 (d, J = 4.9 Hz, 1H), 6.44 (s, 1H), 5.30 (s, 1H), 4.01 (s, 3H), 3.87–3.80 (m, 5H), 3.72 (s, 2H), 3.40 (d, J = 16.5 Hz, 1H), 3.18 (d, J = 5.9 Hz, 1H), 2.79 (s, 1H), 2.30–2.25 (m, 4H), 1.70 (d, J = 5.1 Hz, 2H), 1.57 (d, J = 8.8 Hz, 1H), 1.48 (t, J = 8.0 Hz, 1H), 1.10 (d, J = 10.1 Hz, 1H), 1.00 (d, J = 10.2 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 152.8, 147.8, 147.1, 144.7, 140.1, 139.7, 137.8, 136.5, 130.9, 129.2, 128.9, 128.8, 128.3, 127.7, 127.0, 126.4, 121.1, 120.9, 111.2, 110.2, 64.6, 58.3, 58.2, 56.5, 55.8, 49.8, 49.5, 42.2, 41.5, 32.7, 29.6, 28.4, 21.3 ppm; HRMS (ESI): calcd for C37H38NO2S [M + H]+ 560.2623; found 560.2608.
(Z)-6,7-Dimethoxy-2-methyl-4-(2,3,4,4a-tetrahydro-1H-1,4-methanofluoren-9(9aH)-ylidene)-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline (3p). Yield: 58%, white foam, mp 96–100 °C. 1H NMR (400 MHz, CDCl3) δ 7.44 (s, 1H), 7.31 (d, J = 7.6 Hz, 2H), 7.19 (d, J = 3.8 Hz, 2H), 7.12 (d, J = 7.1 Hz, 3H), 7.07–7.04 (m, 1H), 6.45 (s, 1H), 5.25 (s, 1H), 3.98 (s, 3H), 3.84 (s, 3H), 3.76 (d, J = 16.6 Hz, 1H), 3.45 (d, J = 16.5 Hz, 1H), 3.31 (d, J = 6.1 Hz, 1H), 3.09 (d, J = 6.1 Hz, 1H), 2.68 (s, 1H), 2.34–2.30 (m, 7H), 1.73–1.60 (m, 3H), 1.47–1.42 (m, 1H), 0.98 (d, J = 10.3 Hz, 1H), 0.87 (d, J = 10.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 148.6, 148.3, 147.0, 144.0, 143.6, 137.0, 136.8, 129.2, 128.8, 128.2, 128.1, 127.5, 127.2, 126.1, 124.6, 124.2, 112.0, 109.1, 65.1, 56.1, 55.8, 53.6, 52.3, 51.5, 44.9, 42.8, 42.1, 33.5, 30.8, 27.3, 21.3 ppm; HRMS (ESI): calcd for C33H36NO2 [M + H]+ 478.2746; found 478.2732.
Acknowledgements
One of the authors K. N. gratefully acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi, India for the research fellowship.
References
-
(a) Y. Luo, X. Pan, X. Yu and J. Wu, Chem. Soc. Rev., 2014, 43, 834 RSC;
(b) Y. Yamamoto, Chem. Soc. Rev., 2014, 43, 1575 RSC;
(c) M. Bandini, Chem. Soc. Rev., 2011, 40, 1358 RSC;
(d) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed;
(e) D. D’Souza and T. Müller, Chem. Soc. Rev., 2007, 36, 1095 RSC;
(f) B. Yao, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2012, 51, 12311 CrossRef CAS PubMed.
-
(a) R. Chinchilla and C. Nájera, Chem. Rev., 2014, 114, 1783 CrossRef CAS PubMed;
(b) C. Campbell, R. Greenaway, O. Holton, H. Chapman and E. Anderson, Chem. Commun., 2014, 50, 5187 RSC;
(c) F. Ling, Z. Li, C. Zheng, X. Liu and C. Ma, J. Am. Chem. Soc., 2014, 136, 10914 CrossRef CAS PubMed;
(d) M.-C. Cordonnier, S. Kan, B. Gockel, S. Goh and E. Anderson, Org. Chem. Front., 2014, 1, 661 RSC;
(e) L. F. Tietze, C. Eichhorst, T. Hungerland and M. Steinert, Chem.–Eur. J., 2014, 20, 12553 CrossRef CAS PubMed;
(f) C. Zheng, J. J. Chen and R. Fan, Org. Lett., 2014, 16, 816 CrossRef CAS PubMed;
(g) H. Ohno, Asian J. Org. Chem., 2013, 2, 18 CrossRef CAS PubMed.
-
(a) X.-F. Xia, N. Wang, L. Zhang, X.-R. Song, X.-Y. Liu and Y.-M. Liang, J. Org. Chem., 2012, 77, 9163 CrossRef CAS PubMed;
(b) N. Yoshikai and Y. Wei, Asian J. Org. Chem., 2013, 2, 466 CrossRef CAS PubMed;
(c) M. V. Pham and N. Cramer, Angew. Chem., Int. Ed., 2014, 53, 3484 CrossRef CAS PubMed;
(d) J. Zhao, N. Asao, Y. Yamamoto and T. Jin, J. Am. Chem. Soc., 2014, 136, 9540 CrossRef CAS PubMed.
-
(a) M. Catellani, E. Motti and N. Ca’, Acc. Chem. Res., 2008, 41, 1512 CrossRef CAS PubMed;
(b) E. Motti, F. Faccini, I. Ferrari, M. Catellani and R. Ferraccioli, Org. Lett., 2006, 8, 3967 CrossRef CAS PubMed;
(c) E. Motti, N. Ca’, D. Xu, A. Piersimoni, E. Bedogni, Z.-M. Zhou and M. Catellani, Org. Lett., 2012, 14, 5792 CrossRef CAS PubMed;
(d) F. Faccini, E. Motti and M. Catellani, J. Am. Chem. Soc., 2004, 126, 78 CrossRef CAS PubMed.
-
(a) H. Weinstabl, M. Suhartono, Z. Qureshi and M. Lautens, Angew. Chem., Int. Ed., 2013, 52, 5305 CrossRef CAS PubMed;
(b) L. Jiao, E. Herdtweck and T. Bach, J. Am. Chem. Soc., 2012, 134, 14563 CrossRef CAS PubMed;
(c) D. Candito, D. Dobrovolsky and M. Lautens, J. Am. Chem. Soc., 2012, 134, 15572 CrossRef CAS PubMed;
(d) Z. Qureshi, H. Weinstabl, M. Suhartono, H. Liu, P. Thesmar and M. Lautens, Eur. J. Org. Chem., 2014, 4053 CrossRef CAS PubMed;
(e) V. Narbonne, P. Retailleau, G. Maestri and M. Malacria, Org. Lett., 2014, 16, 628 CrossRef CAS PubMed.
-
(a) H. Liu, M. El-Salfiti and M. Lautens, Angew. Chem., Int. Ed., 2012, 51, 9846 CrossRef CAS PubMed;
(b) P. Zhou, L. Zheng, J. Ma, Y. Ye, X. Liu, P. Xu and Y. Liang, Chem.–Eur. J., 2014, 20, 6745 CrossRef CAS PubMed;
(c) Y. Tanioka and N. Tsukada, J. Org. Chem., 2014, 79, 5301 CrossRef CAS PubMed;
(d) B.-H. Tan and N. Yoshikai, Org. Lett., 2014, 16, 3392 CrossRef CAS PubMed.
-
(a) P. Thansandote, E. Chong, K.-O. Feldmann and M. Lautens, J. Org. Chem., 2010, 75, 3495 CrossRef CAS PubMed;
(b) P.-X. Zhou, Y.-Y. Ye, J.-W. Ma, L. Zheng, Q. Tang, Y.-F. Qiu, B. Song, Z.-H. Qiu, P.-F. Xu and Y.-M. Liang, J. Org. Chem., 2014, 79, 6627 CrossRef CAS PubMed;
(c) D. Chai and M. Lautens, J. Org. Chem., 2009, 74, 3054 CrossRef CAS PubMed.
-
(a) Y. Gao, Y. Huang, W. Wu, K. Huang and H. Jiang, Chem. Commun., 2014, 50, 8370 RSC;
(b) F. Jafarpour, N. Jalalimanesh, M. Teimouri and M. Shamsianpour, Chem. Commun., 2015, 51, 225 RSC;
(c) H. Zheng, Y. Zhu and Y. Shi, Angew. Chem., Int. Ed., 2014, 53, 11280 CrossRef CAS PubMed;
(d) D. Brown, R. Grigg, V. Sridharan, V. Tambyrajah and M. Thornton-Pett, Tetrahedron, 1998, 54, 2595 CrossRef CAS;
(e) J. Mao and W. Bao, Org. Lett., 2014, 16, 2646 CrossRef CAS PubMed;
(f) H. Liu, M. El-Salfiti, D. Chai, J. Auffret and M. Lautens, Org. Lett., 2012, 14, 3648 CrossRef CAS PubMed;
(g) D. Hulcoop and M. Lautens, Org. Lett., 2007, 9, 1761 CrossRef CAS PubMed.
-
(a) Y. Hong, J. Lam and B. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC;
(b) Y.-H. Hsu, Y.-A. Chen, H.-W. Tseng, Z. Zhang, J.-Y. Shen, W.-T. Chuang, T.-C. Lin, C.-S. Lee, W.-Y. Hung, B.-C. Hong, S.-H. Liu and P.-T. Chou, J. Am. Chem. Soc., 2014, 136, 11805 CrossRef CAS PubMed;
(c) R. Hu, N. Leung and B. Tang, Chem. Soc. Rev., 2014, 43, 4494 RSC;
(d) Z. Zhao, J. Lam and B. Tang, J. Mater. Chem., 2012, 22, 23726 RSC.
-
(a) C.-L. Wu, C.-T. Chen and C.-T. Chen, Org. Lett., 2014, 16, 2114 CrossRef CAS PubMed;
(b) S. Lee, T. Yasuda, Y. Yang, Q. Zhang and C. Adachi, Angew. Chem., Int. Ed., 2014, 53, 6402 CrossRef CAS PubMed;
(c) S. Kim, I. Cho, M. Sim, S. Park and S. Park, J. Mater. Chem., 2011, 21, 9139 RSC;
(d) H. Tsuji, C. Mitsui and E. Nakamura, Chem. Commun., 2014, 50, 14870 RSC.
-
(a) M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Tang, J. Mater. Chem., 2010, 20, 1858 RSC;
(b) A. Qin, J. Lam, L. Tang, C. Jim, H. Zhao, J. Sun and B. Tang, Macromolecules, 2009, 42, 1421 CrossRef CAS;
(c) Y. Cai, L. Li, Z. Wang, J. Sun, A. Qin and B. Tang, Chem. Commun., 2014, 50, 8892 RSC;
(d) J. Wang, J. Mei, W. Yuan, P. Lu, A. Qin, J. Sun, Y. Ma and B. Tang, J. Mater. Chem., 2011, 21, 4056 RSC.
-
(a) D. Ding, K. Li, B. Liu and B. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed;
(b) X. Zhang, X. Zhang, B. Yang, Y. Zhang and Y. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 3600 CrossRef CAS PubMed;
(c) H. Shi, J. Liu, J. Geng, B. Tang and B. Liu, J. Am. Chem. Soc., 2012, 134, 9569 CrossRef CAS PubMed.
- A. Nandakumar and P. T. Perumal, Org. Lett., 2013, 15, 382 CrossRef CAS PubMed.
-
(a) A. Nandakumar, S. E. Kiruthika, K. Naveen and P. T. Perumal, Org. Biomol. Chem., 2014, 12, 876 RSC;
(b) K. Naveen, D. Muralidharan and P. T. Perumal, Eur. J. Org. Chem., 2014, 1172 CrossRef CAS PubMed.
- A. Nandakumar, D. Muralidharan and P. T. Perumal, Tetrahedron Lett., 2011, 52, 1644 CrossRef CAS PubMed.
- H. Tong, Y. N. Hong, Y. Q. Dong, Y. Ren, M. Haussler, J. W. Y. Lam, K. S. Wong and B. Z. Tang, J. Phys. Chem. B, 2007, 111, 2000 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 998488. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14621f |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) mine–
mine–![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) ldehyde–
ldehyde–![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) lkyne). Interestingly, the synthesized compounds show a pronounced solid state fluorescence property due to their restriction of intramolecular rotation in the condensed phase.
lkyne). Interestingly, the synthesized compounds show a pronounced solid state fluorescence property due to their restriction of intramolecular rotation in the condensed phase.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) mixture at 10 μM.b Excitation wavelength: 320 nm.
90) mixture at 10 μM.b Excitation wavelength: 320 nm.



