Preparation and hydrophobicity failure behavior of two kinds of fluorine-containing acrylic polyurethane coatings
Abstract
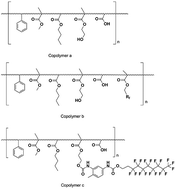
Two kinds of fluorine-containing acrylic copolymers were prepared with an in situ radical polymerization fluorine modification method and a post-polymerization fluorine modification route, respectively. And a kind of common acrylic copolymer as the reference was synthesized. Acrylic polyurethane was prepared using the synthesized acrylic copolymers and a trimer of the hexamethylene diisocyanate curing agent. Different environments including an indoor atmospheric environment, a hygrothermal environment, a different temperature environment, as well as a xenon arc aging environment were employed to investigate the failure behavior of the coatings. Fourier transform infrared spectroscopy (FT-IR) and 19F NMR were employed to characterize the chemical structure of the copolymers. The glass transition temperature (Tg) of the copolymers was tested using differential scanning calorimetry (DSC). The water contact angles of the coatings were monitored during the failure process. The difference in hydrophobicity of the coatings was examined. The thermostability of the coatings was explored using thermogravimetric analysis (TGA). The elemental composition of the coating surface before and after the failure experiment was analyzed using an X-ray photoelectron spectrometer (XPS). The results showed that the fluorine-containing copolymers and the corresponding hydrophobic coatings were prepared as expected. The Copolymer b coating, from the fluorine-containing acrylic copolymer prepared with the in situ polymerization fluorine modification route, exhibited better hydrophobicity in all of the above environments compared with the hydrocarbon acrylic copolymer coating (Copolymer a). The Copolymer c coating, prepared with the fluorine-containing acrylic copolymer via the post-polymerization fluorine modification method, achieved the best hydrophobicity under moderate conditions and failed quickly in hostile environments. The different fluorine modification methods resulted in different failure behaviors.

 Please wait while we load your content...
Please wait while we load your content...