Improved photovoltaic performance in perovskite solar cells based on CH3NH3PbI3 films fabricated under controlled relative humidity
Abstract
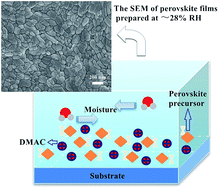
Because organic–inorganic perovskite solar cells are known to be unstable in the presence of moisture, most of the reported high-efficiency perovskite solar cells are fabricated under an inert atmosphere in a glove box. This requirement impedes mass production of organic–inorganic perovskite solar cells (PVSCs). Fabricating dense uniform perovskite thin films with high surface coverage via a single one-step solution process is also a challenge in achieving high-efficiency PVSCs. In this work, we successfully develop a facile, controllable one-step solution processing method to obtain high-quality hybrid-perovskite thin films in ambient atmosphere. The entire preparation process for CH3NH3PbI3 films is conducted in ambient air to investigate the effect of humidity on the molecular structure and crystallization of the hybrid perovskite. It is found that relative humidity (RH) and solvent are crucial factors in determining the final morphology of CH3NH3PbI3 and the photovoltaic performance. The best device efficiency achieved for a solar cell fabricated in ambient atmosphere under a RH of 28% is 16.15%; this PCE value is comparable to that of glove box-based PVSCs. This work puts forth a possible method for the easy mass production of high-performance PVSCs under ambient conditions.

 Please wait while we load your content...
Please wait while we load your content...