Tumor cell responses to carbon dots derived from chondroitin sulfate
Shu-Jun Wang†
ab,
Bei-Bei Wang†bc,
Feng-Wu Bai*ad and
Xiao-Jun Ma*b
aSchool of Life Science and Biotechnology, Dalian University of Technology, Dalian 116023, China. E-mail: fwbai@dlut.edu.cn; Fax: +86-411-84706308
bDivision of Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: maxj@dicp.ac.cn; Fax: +86-411-84379139
cUniversity of the Chinese Academy of Sciences, Beijing 100049, China
dSchool of Life Science and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China
First published on 18th September 2015
Abstract
Mass production of carbon dots (CDs) derived from chondroitin sulfate (CS) was developed by the facile hydrothermal approach for the first time. The CS derived CDs (CSCDs) possessed good dispersibility and water solubility, bright blue and green luminescence, and relative pH- and photo-stable properties. Moreover, the multicolor CSCDs could be efficiently uptaken by SAS cells and exhibited low cytotoxicity. Therefore, the responses of human oral squamous cell carcinoma SAS cells to CSCDs were further investigated by evaluating their proliferation and invasion. Compared to CS, CSCDs not only provided higher efficiency for proliferation of SAS cells, and up-regulated expression of matrix metalloproteinases to mimic extracellular matrix secretion, but also portrayed fluorescence for labeling SAS tumor cells. Hence, the multifunctional CSCDs are expected to have potential for biomedical applications.
Introduction
Carbon dots (CDs) have been the subject of intensive research due to their unique properties such as good water-solubility, low cytotoxicity, eco-friendliness, biocompatibility and particularly their easily tuned optical properties and resistance to photodegradation,1–6 which have been applied in various areas including environmental monitoring, disease diagnostics, bio-imaging and proteomic and genomic studies.7–9 Organic substances such as proteins, amino acids, saccharides and small molecules like citrate acid etc. have been explored as carbonaceous sources for producing CDs.10 However, natural materials are always preferred for this purpose. For instance, CDs have been synthesized from alginate, a negatively charged polysaccharide.11As a natural glycosaminoglycan with good biocompatibility, chondroitin sulfate (CS) is a potential candidate for manufacturing CDs with low cytotoxicity. CS is an anionic linear chain attached onto specific scaffolds, forming a major extracellular matrix (ECM) on the cell surface.12 Despite simplicity of the backbone structure, the CS polymer is complex enough to mediate the cell–cell and cell–ECM interactions that are essential for carrying biological information, and thus determine many biological functions.13,14 For example, CS could strongly influence the proliferation, even migration of malignant tumor cells.15,16 With high anionic activity, CS can interact with various ligands and receptors, and in turn activate signaling pathways,13,17–19 stimulating the growth, migration and metastasis of tumor cells. Previous studies reported that treating tumors with chondroitinase AC led to the reducing growth and invasion of tumor cells.20 Meanwhile, free exogenous CS also exerted effects on the FGF-2-induced proliferative response in melanoma cells.21 CS is the receptor of CD44, which is overexpressed in metastatic cancer tissue, and has been used as anionic component of ternary complexes in drug delivery systems for cancer treatment.22–24 CS polymer has been demonstrated to play an important role in creating microenvironment for protease activation by binding matrix metalloproteinase 2 (MMP2) through the C-domain.25 MMP2 is one of the family members of MMPs, which as zinc-dependent proteolytic enzymes are capable of degrading various components of ECM and bioactive molecules,26 and thus associated with tumor progression, even invasion and metastasis.27,28 Chemically synthesized CS hexasaccharides also enhanced the CD44 cleavage and promoted tumor cell motility in a CD44-dependent manner.24 Therefore CS derived CDs (CSCDs) are expected to remain the functionality of CS, which is vital for tissue engineering research. Moreover, recent progress in nanotechnology has made it possible to design and fabricate biomimetic microenvironment at nanoscale, providing nanoparticles to emulate native ECM.29 Meanwhile, living cells are highly sensitive to local topographic patterns within ECM,29–31 so building nanostructures is a promising approach to mimic native cellular microenvironments.
Herein, a straightforward route using CS as carbonaceous source was developed for manufacturing luminescent CDs with the CS properties of influencing tumor cell proliferation and invasion, which would provide a powerful bio-imaging agent with good biocompatibility to examine the specific mechanism of tumor cell proliferation and invasion.
Experimental part
Materials and cell line
CS and gelatin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Quinine sulfate was purchased from Aladdin. Sodium chloride (>99.5%) and sodium hydroxide (>96%) was purchased from Dalu Chemical Reagent Co. Ltd (Tianjin, China). Phosphate (H3PO4 > 85%) was purchased from Hengxing Chemical Reagent Co. Ltd (Tianjin, China). Boric acid (H3BO3 > 99.5%) was purchased from Bodi Chemical Reagent Co. Ltd (Tianjin, China). Ammonium persulfate, tetramethylethylenediamine (TEMED) and Tris base were purchased from Solarbio (Beijing, China). Acrylamide, glycine and N,N′-methylene-bis-(acrylamide) were purchased from Bio-Rad (California, USA). BCA protein content detection kit was purchased from KeyGEN Biotech Co. Ltd (Nanjing, China). Water used throughout all experiments was purified with the Millipore system.SAS cells from an oral squamous cell carcinoma cell line were kindly donated by Prof. Fujii Masato (National Institute of Sensory Organs, National Tokyo Medical Center, Tokyo, Japan).32
Preparation of CDs
Briefly, CS of 200 mg was dissolved in 10 mL water, and the solution was heated hydrothermally in a stainless steel autoclave at 200 °C for 5 h. The obtained brown solution was cooled down to room temperature, and filtered through a 0.22 μm membrane to remove agglomerated particles. The purified CSCDs were lyophilized and stored at room temperature.Characterization
Transmission electron microscopy (TEM) observation was performed using a FEI TecnaiG2 Spirit at an acceleration voltage of 120 kV. Fourier-transform infrared (FTIR) spectra were recorded by a Burker Vector 22 spectrometer. X-ray diffraction (XRD) measurement was carried out using a PANalytical X'Pert PRO diffractometer (Almelo, Netherlands) in conjunction with Cu Kα radiation (λ = 0.15418 nm). X-ray photoelectron spectrometer (XPS, Thermo Scientific) were used to characterize the chemical composition using an Escalab 250 Xi X-ray photoelectron spectrometer. Absorption and fluorescence spectra were recorded at room temperature with a UV-2550 UV-vis spectrophotometer (Shimadzu, Japan) and Luminescence Spectrometer 55 (Perkin-Elmer), respectively. Fluorescence quantum yield was determined at 360 nm for excitation and 450 nm for emission in 0.10 M H2SO4 solution using quinine sulfate as the reference with a quantum yield of 54%. The effect of pH on the fluorescence (photoluminescence, PL) of CSCDs was examined in Britton–Robinson buffer solutions, which were prepared by 0.04 M each of H3PO4, CH3COOH and H3BO3. Different pH values from 2 to 11 were adjusted by 0.2 M NaOH. Moreover, CSCDs were incubated in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS), FBS-free medium, phosphate buffer solution (PBS) and distilled water, respectively, in a fully humidified incubator at 37 °C with 5% CO2 to see the impact of these conditions.Cell culture
SAS cells were seeded in a 24-well plate containing DME medium with 10% FBS, and incubated in a fully humidified incubator at 37 °C with 5% CO2. The cells cultured at approximately 70–80% confluence were used for further experiment.Fluorescence labeling of SAS cells
CS and CSCDs were dissolved in DMEM medium with final concentrations (mg mL−1) of 0.25, 0.5, 1, 2 and 4, and then filtered by the 0.2 μm Acrodisc syringe filter before being inoculated into cell dishes. Following attachment, SAS cells were incubated with 1 mL CS or CSCDs medium in Φ 20 mm cell culture dishes at 37 °C and 5% CO2 for 4 h. The cells were washed with PBS buffer to remove unbound CS or CSCDs before imaging. Fluorescence imaging studies were performed with laser based point scanning FV 1000 confocal microscope (Olympus, Japan). Oil inserted 100× objective lens were used in the xy-mode with 800 × 800 pixel resolution. Laser beams at 405 nm and 488 nm were used for excitation with emitted light collected between 425–475 nm and 500–600 nm, respectively. Native cells were also imaged under the same conditions as the control.Cell viability analysis
Cell viability was detected using Cell Counting Kit-8 (CCK8) (Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer's instructions. Briefly, CS and CSCDs were dissolved in DMEM, with the final concentrations (mg mL−1) of 0.25, 0.5, 1, 2 and 4. SAS cells were seeded into a 96-well at bottom tissue culture plate with 8 × 103 cells for each well, incubated for 24 h and then treated with various concentrations of CS and CSCDs for 6 d. Cell viability was tested daily. SAS cells were incubated with DMEM containing 10% (v/v) CCK8 at 37 °C for 1 h, and then supernatant was collected to test the absorbance. The absorbance at 450 nm with a reference wavelength of 630 nm was recorded by a microplate reader (Well Scan MK3, Labsystems Dragon, Finland).qRT-PCR analysis
SAS cells cultured with CS or CSCDs at various concentrations were incubated for 6 d at 37 °C and then harvested by trypsin. The total RNA of cells was isolated using RNAiso Plus (TaKaRa, Shiga, Japan) according to the manufacturer's instructions. Reverse transcription (RT) was performed using a PrimeScript RT Reagent Kit (TaKaRa). Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed with SYBR Premix Ex Taq (Takara). qRT-PCR amplification and fluorescence detection were performed using an Mx3000P Real-Time Cycler (Agilent Technologies, Santa Clara, CA, USA). The primers for the qRT-PCR are given in Table 1, which were designed by Takara Biotechnology Co. Ltd (Dalian, China). The results are comparative expression ratio to the control for each sample using the CT method (2−ΔΔCT).| Gene | Sequence (5′ → 3′) | Gene ID | Size (bp) |
|---|---|---|---|
| MMP2 | CTCATCGCAGATGCCTGGAACAGCCTAGCCAGTCGGATTTG | NM_004530 | 167 |
| MMP9 | TGGGCTACGTGACCTATGACATGCCCAGCCCACCTCCACTCCTC | NM_004994.2 | 173 |
| β-Actin | TGGCACCCAGCACAATGAACTAAGTCATAGTCCGCCTAGAAGCA | NM_001101 | 187 |
Zymography
The proteolytic activity of MMPs was assessed by gelatin zymography as previously described.33 SAS cells cultured with various concentrations of CS or CSCDs were incubated for 6 d and then rinsed with a serum-free medium. Condition medium was collected after incubating in the serum-free medium for 24 h and equal amount of protein was used with each sample. Electrophoresis straps indicative of gelatinolytic activity were visualized by staining the gels with coomassie blue (0.5%, w/v), and gelatinolytic activities on the gel zymograph were visualized as clear bands against the blue background.Statistical analysis
Statistical analysis for each sample was calculated using ANOVA. All data were reported as mean ± standard deviation (SD). The significance was tested at p < 0.05.Results and discussion
Optical and physicochemical properties of CS and CSCDs
CS was used to synthesize CDs. The CS aqueous solution was transparent with light yellow even when supplemented at 20 mg mL−1. It was fluorescent under UV light as illustrated in Fig. 1A. After heating hydrothermally for 5 h and filtering out the undissolved substance, a clear yellow solution of CSCDs was obtained, which exhibited bright fluorescence under UV light as illustrated in Fig. 1B. The UV-vis absorption and PL spectra of CS and CSCDs excited at different wavelengths were thus investigated. It was observed that CS had a weak shoulder peak at 320 nm, the typical absorption of n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, while the absorption peak of CSCDs shifted to 315 nm, which was probably due to dehydration and carbonization during hydrothermal treatment. As the excitation wavelength increased from 300 nm to 440 nm with 20 nm increment, the emission of CS and CSCDs also shifted, reaching the peak intensity at 418 nm and 423 nm respectively, both under the excitation wavelength of 340 nm as illustrated in Fig. 1. This excitation wavelength dependent PL emission property was reported previously.34–36 It was believed that besides quantum size effect37,38 and surface defect,38,39 the functional groups on the surface also contributed to PL. To investigate more properties of the CSCDs, further characterization was performed.
O groups, while the absorption peak of CSCDs shifted to 315 nm, which was probably due to dehydration and carbonization during hydrothermal treatment. As the excitation wavelength increased from 300 nm to 440 nm with 20 nm increment, the emission of CS and CSCDs also shifted, reaching the peak intensity at 418 nm and 423 nm respectively, both under the excitation wavelength of 340 nm as illustrated in Fig. 1. This excitation wavelength dependent PL emission property was reported previously.34–36 It was believed that besides quantum size effect37,38 and surface defect,38,39 the functional groups on the surface also contributed to PL. To investigate more properties of the CSCDs, further characterization was performed.
The morphology and size of CSCDs were examined by TEM. As can be seen in Fig. 2, nanodots with an average diameter of 6 nm were developed, validating the formation of CSCDs. The size distribution obtained by measuring totally 80 CSCDs was in accordance with previously reported results.40,41 Although the size exhibited a distribution, almost 65% of CSCDs was less than 10 nm.
 | ||
| Fig. 2 Characterization of CSCDs. (A) Morphology observed with TEM (scale bar: 20 nm), and (B) size distribution of CSCDs. | ||
To investigate the impact of hydrothermal process on CS molecules, XRD characterization was applied. As shown in Fig. 3A, XRD of CSCDs showed peaks at 2θ = 23° and 31° that are assigned to the diffraction of graphitic carbon, which are different from that of CS with one broad peak at 2θ = 23°. This proves that CS decomposed to fractions (carbonization) during the hydrothermal process, which falls in line with TEM results.
Details of structural changes of CS were highlighted by the FTIR. As shown in Fig. 3B, the FTIR spectrum of CS indicated the following functional groups: vibrations of the –OH (3450 cm−1), stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (around 1640 and 1410 cm−1), S
O (around 1640 and 1410 cm−1), S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1250 cm−1) and symmetric and asymmetric stretching vibrations of C–O–C (around 1140 and 1075 cm−1). After the hydrothermal process, N–H associations (3225 cm−1), amido I C
O (1250 cm−1) and symmetric and asymmetric stretching vibrations of C–O–C (around 1140 and 1075 cm−1). After the hydrothermal process, N–H associations (3225 cm−1), amido I C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1680 cm−1), amido II N–H bond (1620 cm−1) and more C–O–C bond (1140 cm−1) were detected, indicating dehydration and decomposition of the CS into CSCDs. The CS and CSCDs both exhibit good hydrosolubility due to their abundant hydrophilic groups (–OH, C
O bond (1680 cm−1), amido II N–H bond (1620 cm−1) and more C–O–C bond (1140 cm−1) were detected, indicating dehydration and decomposition of the CS into CSCDs. The CS and CSCDs both exhibit good hydrosolubility due to their abundant hydrophilic groups (–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O–C).
O, and C–O–C).
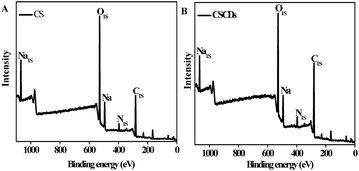
More structural insights could be obtained by XPS spectra, which showed that CS (Fig. 4A) and CSCDs (Fig. 4B) were mainly composed of C (284.27 eV), O (532.04 eV) and N (398.80 eV), and their contents in CS were calculated to be 52.49% (C), 4.45% (N) and 43.06% (O), respectively, while 64.83% (C), 5.27% (N) and 29.91% (O) were detected in CSCDs, indicating the higher doping concentration of nitrogen in the CSCDs. The existence of nitrogen-rich functional groups, as excellent auxochromes, could be the reason for the significant enhancement in PL properties.41
CSCDs possessed pH-dependent luminescence slightly. As shown in Fig. 5A, their PL intensity varied within 25% when the pH changed from acidic to alkaline. The variation corresponded with the protonation and deprotonation of hydroxyl and carboxyl groups, which might cause CSCDs to shift the Fermi level. Under the physiological pH from 5 to 9, the PL intensity of CSCDs was relatively stable. Similar phenomenon was also observed when incubated in various culture conditions illustrated in Fig. 5B, although slight decrease in the PL intensity occurred in the FBS medium, indicating that agglomeration with proteins might occur during the incubation. In addition, CSCDs were photostable, which could be stored under light illumination conditions (data not shown). The quantum yield of CSCDs was 7.2%, higher than that of 6.7% with CS. These properties made CSCDs an ideal material for labeling biological samples.
 | ||
| Fig. 5 Fluorescence response of CSCDs to pH variation (A) and culture conditions without cell inoculation (B). | ||
Cellular imaging of CSCDs
As illustrated in Fig. 6, after co-incubation with 1 mg mL−1 CSCDs for 4 h, the SAS cells showed blue and green fluorescence upon excitation at 405 nm and 488 nm, respectively. This multicolor imaging under different excitation wavelengths highlighted the fluorescent property of CSCDs. However, non-fluorescence was observed in the SAS cells co-incubated with 1 mg mL−1 CS or blank culture medium. Combining the confocal microscopy imaging with the knowledge that particles smaller than 100 nm can be enclosed within endocytic vesicles,42 we speculated that CSCDs might enter the cells via endocytosis route, just like other CDs from different sources.43,44 The fluorescent confocal images of the whole SAS cells incubated with CSCDs indicated that CSCDs could enter into the cells, while CS molecules were blocked from entering cells probably due to their long chains and complex configurations. The cell imaging also showed the dose-dependent property, since cells co-incubated with 0.25 and 0.5 mg mL−1 CSCDs showed weaker fluorescence (data not shown), while CSCDs supplemented at 2 and 4 mg mL−1 exhibited cytotoxicity. | ||
| Fig. 6 Fluorescence image of SAS cells incubated with CSCDs and CS (scale bar: 20 μm). The autofluorescence of cells was minimized by adjusting excitation intensity and PMT parameters. | ||
Dose-dependent impact of CSCDs on the proliferation of SAS cells
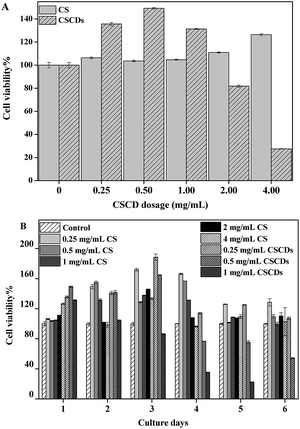
For biomedical use, cytotoxicity is a key factor to be considered.45 For this purpose, the viabilities of SAS cells incubated with CSCDs at 37 °C were evaluated using CCK8 assay with CS as the calibration. As shown in Fig. 7A, the dose-dependent in vitro cytotoxicity of CSCDs on the SAS cells was observed when CSCDs were increased to 2 mg L−1 during 24 h. In contrast, CSCDs under 1 mg L−1 did not impose significant toxicity to SAS cells with long incubation time. As shown in Fig. 7B, more than 50% SAS cells were viable when treated with 0.5 mg mL−1 CSCDs for 6 d. Meanwhile, the results also indicated that CSCDs showed significant promotion effect for the SAS cells, which was time- and dose-dependent.CS, as a component of ECM from which CSCDs were derived, has an effect on tumor cell proliferation. What is interesting is that CSCDs with 0.25 mg mL−1 also exhibited this effect. The result is similar to those previously reported due to the degradation of the ECM proteins which affected the biophysical microenvironment associated with tumor cells,46 and thus CSCDs could be emerged as a particularly powerful regulator of the behavior of tumor cells.47
Invasion properties of SAS with CSCDs
CS polymer has been demonstrated to play an important role in creating microenvironment for protease activation by binding with MMPs.25 Among the family members of MMPs, MMP2 and MMP9, also called gelatinases or type-IV collagenases, are closely connected with cancer progression,28 mainly due to their role in degrading native type V, VII and X collagens, fibronectin, elastin and gelatin.48In order to determine the molecular mechanism underlying the effect of CSCDs on the potential metastasis of SAS cells, the expression of the genes encoding MMP2 and MMP9 in the SAS cell lines were analyzed by qRT-PCR. The results showed that CSCDs supplemented with 0.25 mg mL−1 up-regulated the gene expression, while CS exhibited the role at higher concentrations (Fig. 8A). Low molecular weight CS was reported to enhance CD44 cleavage and promote CD44-dependent cell migration in tumor cells,24 which might be the case with the role of CSCDs in the cell invasion process.
Zymography analysis was performed to quantify the secretion of MMP2 and MMP9, indicating their gelatinolytic activity in the cell culture supernatant collected on day 6 (Fig. 8B). The results illustrated that neither the activity of MMP2 nor MMP9 showed an increase with CSCDs. It is worth noting that CS increased pro-MMP9 amount with the concentrations of 0.5 and 1 mg mL−1, but the active MMP9 had no change. This might be attributed to the change of CS structure during the preparation of CSCDs. The zymography results suggested that CSCDs and CS had no effect on the activities of MMP2 and MMP9 of the two-dimensional cultured SAS cells.
In contrast to the high doses of CSCDs inducing cytotoxicity, these results showed that SAS cells responded to low doses of CSCDs by promoting cell proliferation (Fig. 7B) and improving gene expression for invasion (Fig. 8A), which might be explained as “hormesis”,49 a dose–response process: low concentration improves cell proliferation while high concentration exhibits toxic effect.50,51 This is particularly the case for investigating the toxicological effect of nanoparticles, which is markedly impacted by risk considerations that are based on the assessment of high dose effect and the determination of toxic thresholds at fixed periods of observation.
CS not only promotes cell proliferation, but also improves the expression of invasion related genes,52 which have been broadly applied in simulating the component of ECM in the body.53 Although CDs have been widely utilized owing to their unique physical and chemical properties,54 there is no research so far to combine CS and CDs to investigate their influence on tumor cells. This study demonstrated that SAS cells cultured with CSCDs showed improved cell viability and expression of genes related to tumor invasion. CSCDs might affect the behaviors of the tumor cells through their nanoscale structures as previously reported.30,55,56 These results suggest that CSCDs closely mimic the ECM component on which tumor cells survive in vivo. Additionally, CS can stimulate the growth and differentiation of neurons and prevent normal brain synapses from disintegration after injury,57 and CSCDs could be thus expected for this purpose for the future research while providing fluorescence labelling.
It also points out that the fluorescent CSCDs can be effective tool in investigating interactions of ECM with tumor cells, thus facilitating ECM based therapy in the cancer.
Conclusions
High solubility, good photostability, and low cytotoxicity make CSCDs suitable for biological imaging. Unlike most CDs prepared before, CSCDs prepared from CS retained CS properties to simulate ECM, and thus promoted the invasion and proliferation for SAS cells. On the other hand, compared to CS, CSCDs created nanoscale topography for SAS cells and enhanced the expression of their MMPs genes. As a result, CSCDs combined advantages of CDs and CS together, which was validated in our study for the first time, making them potential multifunctional materials for biomedical applications.Acknowledgements
The authors would like to acknowledge the generous donation of SAS cells from Professor Fujii Masato at the National Institute of Sensory Organs, National Tokyo Medical Center, Tokyo, Japan. We thank Hao Wu for help with the TEM operation. Meanwhile, the assistance of Dr Wei-Ting Yu and Dr Xiu-Li Wang with article writing is highly appreciated.References
- J. C. G. Esteves da Silva and H. M. R. Gonçalves, TrAC, Trends Anal. Chem., 2011, 30, 1327–1336 CrossRef CAS PubMed.
- P.-C. Hsu, P.-C. Chen, C.-M. Ou, H.-Y. Chang and H.-T. Chang, J. Mater. Chem. B, 2013, 1, 1774 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed. Engl., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- R. K. Shukla, J. Mirzaei, A. Sharma, D. Hofmann, T. Hegmann and W. Haase, RSC Adv., 2015, 5, 34491–34496 RSC.
- K. Hola, A. B. Bourlinos, O. Kozak, K. Berka, K. M. Siskova and M. Havrdova, et al., Carbon, 2014, 70, 279–286 CrossRef CAS PubMed.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- B. Kong, A. Zhu, C. Ding, X. Zhao, B. Li and Y. Tian, Adv. Mater., 2012, 24, 5844–5848 CrossRef CAS PubMed.
- Y. Mao, Y. Bao, D. Han, F. Li and L. Niu, Biosens. Bioelectron., 2012, 38, 55–60 CrossRef CAS PubMed.
- G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015 DOI:10.1021/acs.chemrev.5b00008.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- J. F. Y. Fong, S. F. Chin and S. M. Ng, Sens. Actuators, B, 2015, 209, 997–1004 CrossRef CAS PubMed.
- T. Kobayashi, H. Yan, Y. Kurahashi, Y. Ito, H. Maeda and T. Tada, et al., PLoS One, 2013, 8, 350–352 Search PubMed.
- A. P. Asimakopoulou, A. D. Theocharis, G. N. Tzanakakis and N. K. Karamanos, In Vivo, 2008, 22, 385–389 CAS.
- S. Yamada and K. Sugahara, Curr. Drug Discovery Technol., 2008, 5, 289–301 CrossRef CAS.
- C. M. Lee, T. Tanaka, T. Murai, M. Kondo, J. Kimura and W. Su, et al., Cancer Res., 2002, 62, 4282–4288 CAS.
- N. G. Uhlman DL, J. Pathol., 1999, 189, 470–474 CrossRef.
- A. L. M. Joji Iida, J. R. Knutson, L. T. Furcht and J. B. McCarthy, Cancer Biol., 1996, 7, 155–162 CrossRef PubMed.
- Y. L. Yang, C. Sun, M. E. Wilhelm, L. J. Fox, J. Zhu and L. J. Kaufman, Biomaterials, 2011, 32, 7932–7940 CrossRef CAS PubMed.
- S. Y. A. K. Sugahara, Curr. Drug Discovery Technol., 2008, 5, 289–301 CrossRef.
- Y.-Q. L. Elizabeth, M. Denholm and P. J. Silver, Eur. J. Pharmacol., 2001, 416, 213–221 CrossRef.
- D. Nikitovic, M. Assouti, M. Sifaki, P. Katonis, K. Krasagakis and N. K. Karamanos, et al., Int. J. Biochem. Cell Biol., 2008, 40, 72–83 CrossRef CAS PubMed.
- C. D. Nandini and K. Sugahara, Adv. Pharmacol., 2006, 53, 253–279 CAS.
- E. Pantazaka and E. Papadimitriou, Biochim. Biophys. Acta, 2014, 1840, 2643–2650 CrossRef CAS PubMed.
- K. N. Sugahara, T. Hirata, T. Tanaka, S. Ogino, M. Takeda and H. Terasawa, et al., Cancer Res., 2008, 68, 7191–7199 CrossRef CAS PubMed.
- J. Iida, K. L. Wilhelmson, J. Ng, P. Lee, C. Morrison and E. Tam, et al., Biochem. J., 2007, 403, 553–563 CrossRef CAS PubMed.
- L. J. McCawley and L. M. Matrisian, Curr. Opin. Cell Biol., 2001, 13, 534–540 CrossRef CAS.
- S. Curran and G. I. Murray, J. Pathol., 1999, 189, 300–308 CrossRef CAS.
- M. Egeblad and Z. Werb, Nat. Rev. Cancer, 2002, 2, 161–174 CrossRef CAS PubMed.
- J. Shi, A. R. Votruba, O. C. Farokhzad and R. Langer, Nano Lett., 2010, 10, 3223–3230 CrossRef CAS PubMed.
- M. Goldberg, R. Langer and X. Jia, J. Biomater. Sci., Polym. Ed., 2007, 18, 241–268 CrossRef CAS PubMed.
- K. Y. Tsang, M. C. Cheung, D. Chan and K. S. Cheah, Cell Tissue Res., 2010, 339, 93–110 CrossRef CAS PubMed.
- K. Okumura, A. Konishi, M. Tanaka, M. Kanazawa, K. Kogawa and Y. Niitsu, J. Cancer Res. Clin. Oncol., 1996, 122, 243–248 CrossRef CAS.
- P. M. Emma Pirila, T. Salo, E. Koivunen and T. Sorsa, Biochem. Biophys. Res. Commun., 2001, 287, 766–774 CrossRef PubMed.
- X. J. Mao, H. Z. Zheng, Y. J. Long, J. Du, J. Y. Hao and L. L. Wang, et al., Spectrochim. Acta, Part A, 2010, 75, 553–557 CrossRef PubMed.
- D. Pan, J. Zhang, Z. Li, C. Wu, X. Yan and M. Wu, Chem. Commun., 2010, 46, 3681–3683 RSC.
- X. Yang, Y. Zhuo, S. Zhu, Y. Luo, Y. Feng and Y. Dou, Biosens. Bioelectron., 2014, 60, 292–298 CrossRef CAS PubMed.
- D. Chowdhury, N. Gogoi and G. Majumdar, RSC Adv., 2012, 2, 12156–12159 RSC.
- X. Guo, C. F. Wang, Z. Y. Yu, L. Chen and S. Chen, Chem. Commun., 2012, 48, 2692–2694 RSC.
- A. Konwar, N. Gogoi, G. Majumdar and D. Chowdhury, Carbohydr. Polym., 2015, 118, 238–245 CrossRef PubMed.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960–9963 CrossRef CAS PubMed.
- B. Shi, L. Zhang, C. Lan, J. Zhao, Y. Su and S. Zhao, Talanta, 2015, 142, 131–139 CrossRef CAS PubMed.
- K.-I. Ogawara, M. Yoshida, K. Furumoto, Y. Takakura, M. Hashida and K. Higaki, et al., J. Drug Targeting, 1999, 7, 213–221 CrossRef CAS PubMed.
- M. Algarra, M. Perez-Martin, M. Cifuentes-Rueda, J. Jimenez-Jimenez, J. C. Esteves da Silva and T. J. Bandosz, et al., Nanoscale, 2014, 6, 9071–9077 RSC.
- B. Han, W. Wang, H. Wu, F. Fang, N. Wang and X. Zhang, et al., Colloids Surf., B, 2012, 100, 209–214 CrossRef CAS PubMed.
- H. U. Lee, S. Y. Park, E. S. Park, B. Son, S. C. Lee and J. W. Lee, et al., Sci. Rep., 2014, 4, 4665 Search PubMed.
- A. R. Anderson, A. M. Weaver, P. T. Cummings and V. Quaranta, Cell, 2006, 127, 905–915 CrossRef CAS PubMed.
- A. Pathak and S. Kumar, Integr. Biol., 2011, 3, 267–278 RSC.
- R. M. Senior, G. L. Griffin, C. J. Fliszar, S. D. Shapiro, G. I. Goldberg and H. G. Welgus, J. Biol. Chem., 1991, 266, 7870–7875 CAS.
- I. Iavicoli, E. J. Calabrese and M. A. Nascarella, Dose-Response, 2010, 8, 501–517 CrossRef CAS PubMed.
- E. J. Calabrese and L. A. Baldwin, Hum. Exp. Toxicol., 2002, 21, 91–97 CAS.
- M. A. Nascarella and E. J. Calabrese, Dose-Response, 2012, 10, 344–354 CrossRef CAS PubMed.
- P. Frankel, C. Pellet-Many, P. Lehtolainen, G. M. D'Abaco, M. L. Tickner and L. Cheng, et al., EMBO Rep., 2008, 9, 983–989 CrossRef CAS PubMed.
- W. Schneiders, C. Rentsch, S. Rehberg, S. Rein, H. Zwipp and S. Rammelt, Mater. Sci. Eng., C, 2012, 32, 1926–1930 CrossRef CAS PubMed.
- M. P. Sk, S. K. Sailapu and A. Chattopadhyay, ChemPhysChem, 2015, 16, 723–727 CrossRef CAS PubMed.
- H. Mao, N. Kawazoe and G. Chen, Colloids Surf., B, 2015, 126, 63–69 CrossRef CAS PubMed.
- S. P. Pathi, D. D. W. Lin, J. R. Dorvee, L. A. Estroff and C. Fischbach, Biomaterials, 2011, 32, 5112–5122 CrossRef CAS PubMed.
- S. E. Tully, R. Mabon, C. I. Gama, S. M. Tsai, X. Liu and L. C. Hsieh-Wilson, J. Am. Chem. Soc., 2004, 126, 7736–7737 CrossRef CAS PubMed.
Footnote |
| † Shujun Wang and Beibei Wang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |