DOI:
10.1039/C5RA14550C
(Paper)
RSC Adv., 2015,
5, 86685-86696
Synthesis, characterization and optoelectronic investigations of bithiophene substituted 1,3,4-oxadiazole derivatives as green fluorescent materials†
Received
22nd July 2015
, Accepted 8th October 2015
First published on 8th October 2015
Abstract
A series of novel unsymmetrical bithiophene substituted oxadiazole derivatives 2(a–e) were designed and synthesised by employing palladium catalysed Suzuki cross coupling reaction. These bipolar molecules consist of bithiophene as an electron donor unit (D) and electron transporting oxadiazole as acceptor unit (A). The structural integrity of all the new compounds was confirmed by 1H NMR, 13C NMR and GC-MS analysis. The photophysical and electrochemical properties have been studied in detail using UV-Vis absorption, fluorescence spectroscopy and CV measurements. All compounds emit intense green fluorescence with good quantum yields. Density functional theory computations have been carried out to understand the structure–property relationship, the computed values are found to be in good agreement with the experimental results. The results demonstrated that the novel bithiophene containing oxadiazole derivatives could play an important role in organic optoelectronics.
Introduction
Photoluminescence (PL) of organic compounds has been studied extensively to develop thin, efficient, and stable devices with wide viewing angles and fast response. In recent years, PL of organic materials with excellent characteristics has been exploited for the development and commercialization of display materials. The optoelectronic properties of conjugated polycyclic aromatic compounds plays a significant role in photonic and electronic devices such as organic light-emitting diodes (OLEDs) which exhibit a great potential to revolutionize display technologies in the field of organic electronics. The most successful and suitable OLEDs are good substitutes for liquid crystal-based devices,1 because of their comparatively low power consumption, compatibility with large area and flexible substrates, and tuneability by molecular structure modification.2,3 A great deal of research work has been focused on the development of visible light emitting OLEDs for display and lighting applications.4–7 Compared to other display technologies, OLEDs show their own unique advantages like, easy processing, self-luminescence, high brightness, high efficiency, low drive voltage, wide viewing angle, high contrast and high-speed response. OLEDs are currently used in long-lived and highly efficient colour displays and also hold unique applications in biological and chemical sensing,8,9 high-density information storage10 and full-colour light-emitting displays.11,12
For the purpose of practical use, they still have several unsolved problems such as operational stability of the devices and colour shift after operation. Some of these problems could be solved by modification of structural backbone of organic compounds. In designing OLEDs, a luminescent material is required in which electron and hole are recombined resulting in the emission of light.13–16 By employing donor/acceptor strategy one can improve the device efficiency and operational lifetime along with fluorescence properties.17 In order to achieve high fluorescence, small bipolar molecules can be designed by incorporating electron-withdrawing groups like 1,3,4-oxadiazole, phosphineoxide, triazine and electron-donating groups like carbazole, diphenylamine etc.18–22 The performance of diodes is closely governed by the number of hole (HT) and electron transporting (ET) moieties. Among all fluorescent heterocyclic ring systems, the molecules containing 1,3,4-oxadiazole, play a crucial roles like excellent electron-acceptor, high thermal stability, high quantum yields. They are also used as electron-transporting/hole-blocking materials in OLEDs.23,24 Indeed, oxadiazole units advantageously restrict π-conjugation to afford materials with deeply lying highest occupied molecular orbital's (HOMOs) such that the triplet energies (ETs) are high.25–27
In the present investigation, we have designed 1,3,4-oxadiazole derivatives with focus on the HOMO–LUMO gap in donor–acceptor (D/A) systems which can be tuned either by altering the strength of D/A units or by extending π conjugation. The incorporation of electron deficient 1,3,4-oxadiazole ring with electron rich bithiophene as a donor results in strong intramolecular charge transfer (ICT) chromophores.28,29 In view of this, we have designed and synthesized a novel series of unsymmetrical small bipolar organic molecules 2(a–e) (Fig. 1) with bithiophene substituent as an electron donor, which is connected through a phenyl spacer with para linkages. The introduction of the bithiophene moiety extends the π–π conjugation and oxadiazole enhances the electron transporting capability. We have studied the optoelectronic properties such as UV-Vis/fluorescence spectra, quantum yields and HOMO–LUMO calculations. Additionally, we studied the effect of solvents (solvatochromic behavior) to support strong intramolecular charge transfer property of these compounds. Further, the electrochemical property of the compounds has been studied by cyclic voltammetry (CV). The thermal properties of compounds were investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The systematic investigation of photophysical, electrochemical and thermal properties of newly synthesised compounds were performed with a view to examine their suitability in developing OLEDs.
 |
| | Fig. 1 UV-Vis absorption spectra of compounds 2(a–e) in ethanol at room temperature, compared with reference (coumarin 480). | |
Results and discussion
Synthesis and characterization
Designed title compounds 2(a–e) are illustrated in Scheme 1. A relatively simple and efficient synthetic protocol was employed for the synthesis of compounds 2(a–e) as depicted in Scheme 2. The required precursor 4-bromobenzohydrazide was obtained by the esterification of 4-bromobenzoic acid followed by treating with hydrazine hydrate.30,31 The key intermediates 1(a–e) were synthesized by treatment of 4-bromobenzohydrazide with various aromatic carboxylic acids in refluxing POCl3 and intermediates 1b, 1c and 1e were reported in the literature.32–34 Subsequent Pd-catalyzed Suzuki cross-coupling reaction between 1(a–e) and 5′-hexyl-2,2′-bithiophene-5-boronic acid pinacol ester afforded the target compounds in 80–85% yields. At the second position of oxadiazole the bithiophene group is kept constant and we have varied in the substituents in the fifth position with different groups such as p-tert-butylphenyl, thienyl, biphenyl, pentafluorophenyl and 2-napthyl to modify their spectral properties. The resulting bipolar molecules were purified by column chromatography on silica gel followed by recrystallization in ethanol before spectral characterization. All these compounds 2(a–e) are amorphous in nature and can be stored under ambient conditions for longer time without any detectable decomposition. All these compounds are readily soluble in common organic solvents like EtOH, CHCl3, DCM and THF etc. Their structural identities and purities were confirmed by 1H NMR, 13C NMR, IR and MS and were found to be in good agreement with the proposed structures.
 |
| | Scheme 1 Chemical structures of target compounds 2(a–e). | |
 |
| | Scheme 2 Synthetic route for the target compounds 2(a–e). | |
Photophysical properties
UV-Vis absorption and photoluminescence (PL) spectra. All the derivatives are soluble in common organic solvents, such as tetrahydrofuran (THF), dichloromethane, chloroform and ethanol. Fig. 1 shows UV-Vis absorption spectra of the synthesized compounds in ethanol (HPLC grade). The maximum absorption wavelengths (λabsmax) of 2a, 2b, 2c, 2d, and 2e are 387, 386, 386, 391 and 381 nm, respectively. Molecules 2(a–e) exhibit two distinct peaks in the absorption spectra, even though there was no conjugation break at the phenyl–thiophene bond of the compounds, we observed a strong absorption bands at shorter wavelengths mainly because of π–π* transition of local electrons present in the bithiophene moiety of the compound (electron-donor) and less intense absorption bands at longer wavelengths at 350–450 nm were assigned to the π–π* transition of whole segment. In the series 2(a–e), the derivative 2e shows least λabsmax of 381 nm and 2d exhibited highest λabsmax of 391 nm (Table 1).
Table 1 Summary of optical and thermal properties of π-conjugated molecules 2(a–e)
| Compounds |
Absorption λabsmaxa (nm) |
|
Emission |
Stoke shift Δλ (nm) |
Quantum yield |
Tg/Tm/Tc/T5dd [°C] |
| λemimaxb (nm) |
λemimaxc (nm) |
|
| The absorption spectra were measured in ethanol at 10 μM concentration. The emission spectra were measured in ethanol at 10 μM concentration. Ref – coumarin 480.36 The emission spectra were measured in solid state film. Obtained from DSC and TGA measurements; Tg-glass transition temperature, Tm-melting temperature and Tc-crystallization temperature. |
| 2a |
387 |
461 |
519 |
74 |
0.41 |
87.36/141.15/—/267 |
|
| 2b |
386 |
462 |
487 |
76 |
0.44 |
87.49/139.38, 158.58/—/228 |
|
| 2c |
386 |
463 |
475 |
77 |
0.35 |
87.74/196.13/—/268 |
|
| 2d |
391 |
466 |
503 |
75 |
0.60 |
86.86/155.13/—/214 |
|
| 2e |
381 |
466 |
465 |
85 |
0.45 |
—/248.38/—/235 |
|
| Ref |
389 |
457 |
— |
68 |
0.95 |
—/—/—/— |
|
Photoluminescence (PL) spectra (Fig. 2(a)) could provide good deal of information on the electronic structure of the synthesised molecules. A marginal bathochromic shift observed in emission spectra of 2(a–e) might be attributed to different aryl/heteroaryl groups at the fifth position of oxadiazole unit. Even though there was not much deference with respect to absorption and fluorescence maxima, we observed significant difference in the intensity of absorption and emission spectra among these compounds (Fig. 1 and 2(a)) due to different aryl/heteroaryle groups at the fifth position of the oxadiazole moiety. The emission maxima of 2a, 2b and 2c in ethanol were 461, 462 and 463 nm respectively, while 2d and 2e were red-shifted to 466 nm (Table 1). In addition, emission spectra of compounds 2(a–e) in solid state film were measured by exciting at 385 nm (Fig. 2(b)) to examine the emission properties of these new compounds. The emission maxima for all compounds were summarised in Table 1.
 |
| | Fig. 2 (a) Emission spectra of compounds 2(a–e) in ethanol at room temperature, compared with reference (coumarin 480). (b) Emission spectra of compounds 2(a–e) in solid state film when excited at λex = 385 nm. | |
The Stoke shift, indicating the extent of the bathochromic shift of the fluorescence maximum (λemimax) compared to the absorption maxima (λabsmax), is in the range of 74–85 nm. There was a substantial increment in Stoke shift from 2a to 2e except for 2d. Compound 2a & 2e, respectively, have shown smaller and larger Stoke shifts among the five compounds (Table 1).
Quantum yield (Φ) calculations. The radiative quantum yield is an important quantity in molecular chemistry. Quantum yields provide important information regarding excited electronic states, radiationless transitions, and coupling of electronic to vibronic states. Fluorescence quantum yields (Φ) of 2(a–e) were measured in ethanol at room temperature by comparison with a standard dye coumarin 480 (2,3,5,6-1H,4H-tetrahydro-8-methylquinolizino-[9,9a,1-gh]-coumarin) of known quantum yield (Φ) using the eqn (1).35 The compounds 2(a-b), 2(c-d) and 2e were excited at 386 nm, 390 nm and 382 nm respectively.| |
 | (1) |
Where I is the integrated intensity, OD is the optical density and n is the refractive index, the subscript R refers to the reference fluorophore of known quantum yield. The quantum yields of all the compounds are in the range of 0.35 to 0.60 (Table 1). There is a marginal increase in quantum yield from 2a to 2b, may be due to thiophene ring at the fifth position of oxadiazole in 2b. The compound 2d has shown higher quantum yield of 0.60 and 2c has shown comparatively least quantum yield among five compounds.
Optical band gap. In organic molecules, the energy levels of the electronic states correspond to the energy carried by UV or visible radiation. At resonance, the molecules can absorb quantified energy transported by the electromagnetic radiation, and promote an electron from the low-energy molecular orbital to higher energy molecular orbital.37 These transitions can be measured using a UV-Vis spectrophotometer. The optical band (Eoptg) corresponds to the energy of the long wavelength edge of the exciton absorption band.38 The optical band gap values (Eoptg) were approximated from the onset of the low energy side of the absorption spectra (λonset, solution) to the baseline according to the eqn (2) and are presented in (Table 2).| |
 | (2) |
Table 2 Summary of solvent effect on molecules 2(a–e) a
| Solvent |
2a |
2b |
2c |
2d |
2e |
| λa |
λf |
λa |
λf |
λa |
λf |
λa |
λf |
λa |
λf |
| λa: absorption maxima in nm. λf: emission maxima in nm. |
| Methanol |
381 |
474 |
380 |
474 |
389 |
483 |
384 |
501 |
386 |
481 |
| Ethanol |
387 |
461 |
386 |
462 |
386 |
463 |
391 |
466 |
381 |
466 |
| Propanol |
376 |
464 |
361 |
449 |
390 |
455 |
367 |
493 |
392 |
470 |
| Butanol |
383 |
469 |
382 |
467 |
390 |
461 |
387 |
493 |
386 |
469 |
| Pentanol |
384 |
468 |
381 |
466 |
389 |
461 |
387 |
489 |
386 |
466 |
| Toluene |
385 |
460 |
383 |
459 |
391 |
462 |
388 |
464 |
389 |
448 |
| DMSO |
388 |
472 |
388 |
472 |
395 |
478 |
391 |
506 |
390 |
483 |
| DMF |
384 |
472 |
386 |
473 |
391 |
484 |
386 |
467 |
384 |
486 |
| Chloroform |
385 |
462 |
384 |
462 |
390 |
463 |
391 |
466 |
387 |
461 |
| DCM |
385 |
462 |
384 |
463 |
389 |
463 |
389 |
473 |
388 |
465 |
Solvent effect. The solvatochromic behaviour of compounds 2(a–e) have been studied in series of alcohols (methanol–pentanol) and some general solvents like toluene, dimethyle sulfoxide (DMSO), dimethyleformamide (DMF), chloroform and dichloromethane (DCM) with a view to understand the intramolecular charge transfer effect (ICT) as summarised in Table 2 and Fig. 3 & 4. This solvatochromic behaviour gives the effect of solvents on absorbance and fluorescence maxima through blue or red shifts in compounds 2(a–e). The study of solvent effect based on spectral properties of all compounds in solutions was carried out by using the spectral position in the solvents mentioned here.39 As the shift in λmax values with solvent type reflects molecular interactions. The spectral position of a compound in a variety of solvents reveals interesting results. The absorption bands of solute molecules in different solvents with varying polarity have shown bathochromic as well as hypsochromic shifts. These shifts arise depending on the way a solute molecule interacts with solvent. The solute molecule finds itself in a cavity inside the solvent, resulting in a net stabilization of their ground state. During strong intramolecular charge transfer, if the dipole moment of solute increases, the excited state is formed inside the solvent cavity which is surrounded by partly oriented solvent dipoles. The net stabilization of the excited state as compared to the ground state with increasing solvent polarity results in bathochromic shift. If there is a decrease in dipole moment of solute during the intramolecular charge transfer, the excited state is formed in a strained solvent cavity of oriented dipoles not correctly disposed for its efficient stabilization. The energy of the ground state lowered more than that of the excited state with increasing solvent polarity results in a hypsochromic shift.
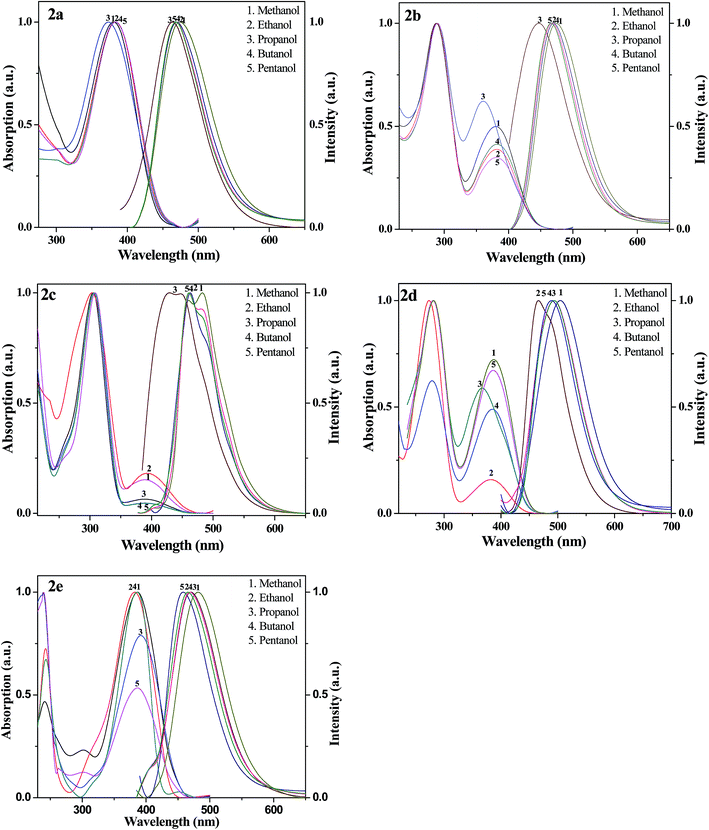 |
| | Fig. 3 Typical absorption and fluorescence spectra of compounds 2(a–e) in series of alcohols. | |
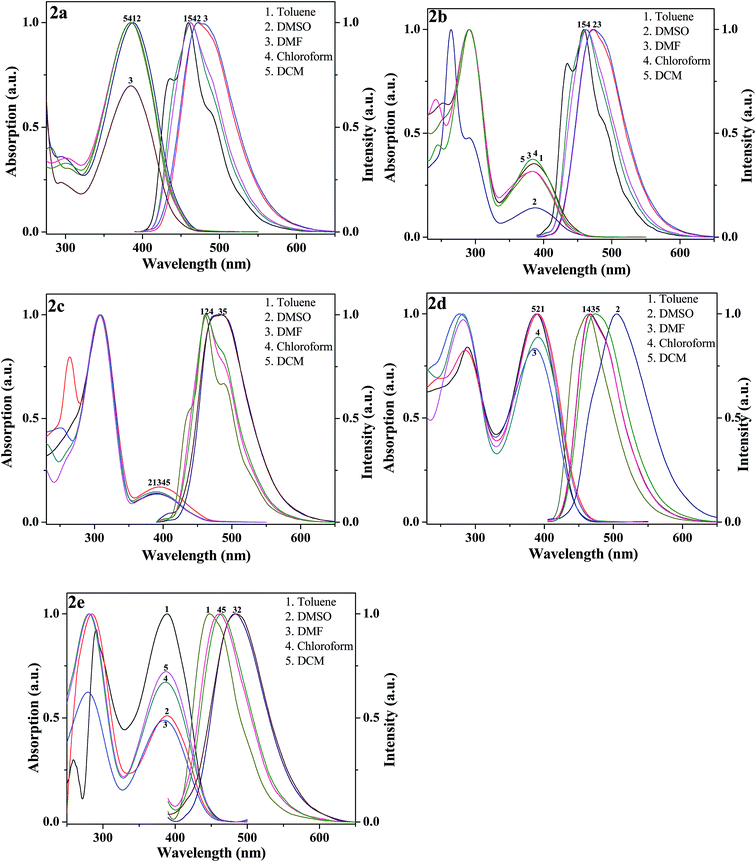 |
| | Fig. 4 Typical absorption and fluorescence spectra of compounds 2(a–e) in general solvents. | |
Electrochemical properties
The electrochemical properties of compounds 2(a–e) were explored by cyclic voltammetry (CV). Further HOMO–LUMO energy levels of these molecules were calculated from cyclic voltammetric measurements. HOMO–LUMO band gap is derived from the difference between HOMO and LUMO energy levels. HOMO energy levels were calculated using eqn (3).40,41| | |
HOMO = −[Eonsetox + 4.44] (eV)
| (3) |
The LUMO energy levels were estimated by using eqn (4) and were compared with those of coumarin 480 (Table 3). These results revealed the facile reversible redox behaviour of the synthesized compounds and hence they can be applied as bipolar transport materials for electroluminescence applications.
| | |
LUMO = [HOMO + Eoptg] (eV)
| (4) |
Table 3 Electrochemical data of compound 2(a–e)
| Compound |
Eonsetoxa (eV) |
HOMOb (eV) |
LUMOc (eV) |
Eoptg (eV) |
| Oxidation potential relative to Ag/AgCl electrode. Calculated HOMO from the onset oxidation potentials of the compounds HOMO = −[Eonsetox + 4.44] (eV). Estimated LUMO using empirical equations LUMO = (HOMO + Eoptg) (eV). |
| 2a |
0.5 |
−5.3 |
−2.49 |
2.81 (441) |
| 2b |
0.6 |
−5.4 |
−2.60 |
2.80 (442) |
| 2c |
0.5 |
−5.3 |
−2.59 |
2.71 (457) |
| 2d |
0.8 |
−5.6 |
−2.82 |
2.78 (446) |
| 2e |
0.5 |
−5.3 |
−2.45 |
2.85 (435) |
| Ref |
0.35 |
−5.15 |
−2.25 |
2.90 (427) |
The trend observed in the optical band gap values exhibited in the order 2e > 2a > 2b > 2d > 2c. This reveals that the optical band gap values were significantly fluctuating from 2(a–e). The biphenyl derivative (2c) has shown the lower optical band gap of 2.71 eV and this was due to the extended conjugation of biphenyl system at the fifth position of oxadiazole unit. The difference in the optical band gap was observed between pentafluorophenyl (2d) and naphthyl derivatives (2e), in which (2d) showed comparatively lesser band gap than compound (2e). Further, it was observed that the electron-withdrawing pentafluorophenyl based compound 2d has exhibited deepest HOMO level compared to all other derivatives (Fig. 5).
 |
| | Fig. 5 CV curves of compounds 2(a–e) were measured in CH2Cl2 in the presence of nBu4NPF6 at a scan rate of 100 mV s−1. | |
Thermal properties
The ability of a material to form morphologically stable films is an important requirement for the successful operation of OLEDs. The thermal and morphological stabilities of compounds 2(a–e) were determined by differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). The results are shown in (Fig. 9) and summarised in (Table 1). DSC was performed in the temperature range 25–300 °C under nitrogen with a heating rate of 10 °C min−1. The phase transition properties of these compounds were analyzed by DSC, it reveals that, compounds 2a, 2b, 2c and 2d are semicrystalline, and its DSC curve shows an endothermic baseline shift related to the glass transition temperature (Tg) at 87.36 °C, 87.49 °C, 87.74 °C and 86.86 °C respectively. It is important to notice that the glass transition (Tg) temperatures of compounds 2(a–d) are higher than those of the most popular oxadiazole-based electron-transporting materials, such as, 2-(4-biphenylyl)-5-(4-tert-butylphenyl)-1,3,4-oxadiazole (PBD, Tg = 60 °C), 1,3-bis[(4-tert-butylphenyl)-1,3,4-oxadiazolyl]phenylene (OXD-7, Tg = 77 °C) and are higher than the non-bipolar host-emitters that contain oxadiazoles (BOBP, Tg = 45 °C; TPOs and PPOs, Tgs up to 121 °C).25 Followed by the glass transition temperature (Tg) compounds 2(a–d) shows an endothermic melting peaks at temperature around 139–196 °C. Compound 2e is a crystalline material with only one sharp endothermic melting peak at 248.38 °C being detected in the thermogram. Exothermic peaks were not observed at higher temperature resulting from crystallisation, indicates compounds with excellent amorphous glass state stability. The TGA results suggested that all compounds were thermally stable with 5% weight loss temperatures (T5d) of compounds 2(a–e) under N2, were ranged in 214–268 °C, demonstrating good thermal stability.
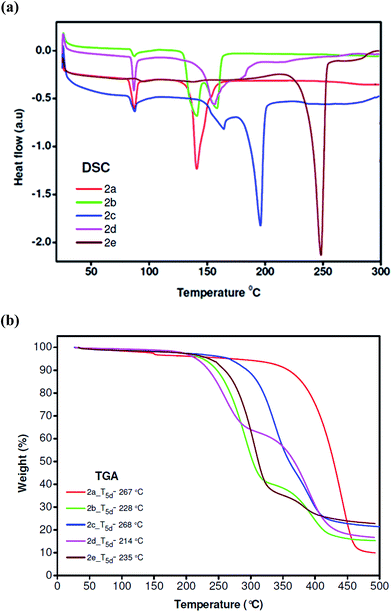 |
| | Fig. 9 (a) DSC and (b) TGA thermograms of the compounds 2(a–e) measured at a heating rate of 10 °C min−1 under N2. | |
Conclusions
We have successfully designed and synthesized novel series of bithiophene substituted oxadiazoles by employing a relatively simple synthetic protocol. Structures of these newly synthesized compounds were established by their analytical and spectral data. We have studied the optoelectronic properties like UV-Vis spectra, fluorescence emission spectra and quantum yield calculations. Additionally HOMO–LUMO calculations are performed. All compounds fluoresce in the green range with high quantum yields. The fluorescence quantum yield was found to be appreciable for pentafluorophenyl derivative (2d) of 0.60 compare to reference (coumarin 480). We have calculated HOMO, LUMO and energy band gap values for all the compounds and optical band gaps obtained from absorption thresholds are in good agreement with the band gaps obtained from DFT calculations. The results revealed that the bithiophene-substituted oxadiazole derivatives could play an important role in optoelectronic applications.
Experimental section
Physical measurements
All chemicals are reagent/analytical grade and used without further purification. 5′-Hexyl-2,2′-bithiophene-5-boronic acid pinacol ester was purchased from Sigma-Aldrich. Melting points were determined by open capillary method and are uncorrected. The IR spectra were recorded on Nicolet Impact 410 FT IR spectrophotometer using KBr pellets. 1H and 13C NMR were recorded on Bruker 400 MHz FT NMR spectrometer in CDCl3 by using TMS as internal standard. Chemical shifts are reported in ppm downfield (δ) from TMS. Mass spectra were recorded using Shimadzu GCMS-QP2010S. Elemental analyses were performed on a Vario III elemental analyzer. The UV-Vis absorption spectra were recorded using JASCO UV-Vis NIR Spectrophotometer (Model V-670). Photoluminescence spectra were measured using Spectro-fluorometer (JY Horiba, Floromax-4). Fluorescence quantum yields (Φ) of the compound solutions were estimated by comparing wavelength-integrated photoluminescence (PL) intensity of the compound solutions with that of the reference. The DFT calculations were carried out using Gaussian-09. Cyclic voltammetry (CV) was carried out in a three-electrode cell with a Pt counter electrode, an Ag/AgCl reference electrode, and a glassy carbon working electrode at a scan rate of 100 mV s−1 with 0.1 M nBu4NPF6 as the supporting electrolyte, in anhydrous dichloromethane solution. DSC and TGA were performed with a TA Instruments DSC Q20 V24.10 Build 122 and TA Instruments SDT Q600 V20.9 Build 20, respectively, under nitrogen with heating rates of 10 °C min−1.
Materials and synthesis
All solvents were freshly distilled over appropriate drying reagents prior to use. All starting materials were purchased from Sigma Aldrich and were used without further purification. The required intermediate 4-bromobenzhydrazide and compounds 1(a–e) were synthesized according to the published procedures.30 The catalyst Pd(dppf)2Cl2 and 5′-hexyl-2,2′-bithiophene-5-boronic acid pinacol ester were purchased from Sigma-Aldrich.
General procedure for the synthesis of final compounds 2(a–e)
To a mixture of 4-bromo phenyl 1,3,4-oxadiazole 1(a–e) (0.5 g, 1.39 mmol), 5′-hexyl-2,2′-bithiophene-5-boronic acid pinacol ester (0.25 g, 1.54 mmol), Pd(dppf)2Cl2 (0.051 g, 0.07 mmol), as a catalyst were added to a mixture of 1,4 dioxane (10 mL) and aqueous 2 M K2CO3 (5 mL). The reaction mixture was heated for 6–8 h at 85–90 °C under nitrogen. The progress of the reaction was frequently monitored by TLC, the solution was extracted with CH2Cl2 and the organic layer was washed with water (25 mL) and brine solution (25 mL), and then dried over anhydrous Na2SO4. Organic solvent was removed completely under reduced pressure, the crude residue was purified by flash column chromatography on silica gel using plane chloroform as an eluting solvent to obtain desired compound in 80–85% yield.
2-(4-tert-butylphenyl)-5-(4-(5-(5-hexylthiophen-2-yl)thiophen-2-yl)phenyl)-1,3,4-oxadiazole (2a). Yield: 82%, colour: light green. MP = 143–145 °C. 1H NMR (500 MHz, CDCl3) δ: 8.14 (d, J = 7.6 Hz, 2H), 8.09 (d, J = 7.7 Hz, 2H), 7.74 (d, J = 7.6 Hz, 2H), 7.58 (d, J = 7.8 Hz, 2H), 7.31 (d, J = 29.3 Hz, 1H), 7.07 (t, J = 19.5 Hz, 2H), 6.71 (d, J = 15.4 Hz, 1H), δ 2.83 (t, J = 7.2 Hz, 2H), 1.71 (d, J = 6.9 Hz, 2H), 1.38 (m, 6H), 0.93 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 164.130, 155.349, 140.702, 137.216, 134.377, 127.455, 126.793, 126.065, 125.688, 125.018, 124.001, 123.768, 123.553, 122.460, 35.118, 31.586, 31.575, 31.149, 30.228, 29.787, 22.599, 14.107; MS m/z calculated for C32H34N2OS2 526.76, found 526 (M+); anal. calcd (%) for C32H34N2OS2: C 72.96, H 6.51, N 5.32. Found: C 72.90, H 6.07, N 5.25.
2-(4-(5-(5-hexylthiophen-2-yl)thiophen-2-yl)phenyl)-5-(thiophen-2-yl)-1,3,4-oxadiazole (2b). Yield: 85%, colour: green. MP = 158–160 °C. 1H NMR (500 MHz, CDCl3) δ: 7.91 (d, J = 2.6 Hz, 4H), 7.61 (3H), 7.20 (d, J = 2.3 Hz, 1H), 7.10 (d, J = 20.2, 2.0 Hz, 1H), 6.80 (d, J = 20.2, 2.0 Hz, 1H), 6.71 (d, J = 20.2, 2.0 Hz, 1H), 2.80 (2H), 1.72–1.34 (m, 8H), 0.92 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 164.5, 161.2, 143.3, 138.1, 136.7, 134.2, 133.8, 132.2, 128.5, 128.1, 127.9, 127.6, 126.8, 126.2, 125.9, 125.5, 43.1, 32.1, 31.9, 29.1, 22.8, 14.1; MS m/z calculated for C26H24N2OS2 476.68, found 476 (M+); anal. calcd (%) for C26H24N2OS3: C 65.51, H 5.07, N 5.88. Found: C 65.48, H 5.03, N 5.84.
2-(4-(5-(5-hexylthiophen-2-yl)thiophen-2-yl)phenyl)-5-(biphenyl)-1,3,4-oxadiazole (2c). Yield: 78%, colour: light brown. MP = 198–200 °C. 1H NMR (500 MHz, CDCl3) δ: 8.17 (d, J = 7.6 Hz, 2H), 8.11 (d, J = 8.0 Hz, 2H), 7.72–7.61 (m, 4H), 7.45–7.33 (m, 4H), 7.31 (d, J = 29.3 Hz, 1H), 7.25 (t, J = 7.4 Hz, 1H), 7.07 (t, J = 19.5 Hz, 2H), 6.71 (d, J = 15.4 Hz, 1H), δ 2.83 (t, J = 7.2 Hz, 2H), 1.71 (d, J = 6.9 Hz, 2H), 1.38 (m, 6H), 0.93 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 164.5, 143.1, 138.4, 136.7, 136.6, 134.2, 133.6, 129.3, 128.0, 127.9, 127.7, 126.2, 125.1, 43.3, 32.1, 31.9, 29.1, 22.8, 14.1; MS m/z calculated for C34H30N2OS2 546.74, found 546 (M+); anal. calcd (%) for C34H30N2OS2: C 74.96, H 5.53, N 5.12. Found: C 74.89, H 5.47, N 5.15.
2-(4-(5-(5-hexylthiophen-2-yl)thiophen-2-yl)phenyl)-5-(perfluorophenyl)-1,3,4-oxadiazole (2d). Yield: 75%. colour: dark green. MP = 160–163 °C. 1H NMR (500 MHz, CDCl3) δ: 8.08 (dd, J = 8.0 Hz, 1H), 7.74 (t, J = 10.8 Hz, 1H), 7.38–7.28 (m, 1H), 7.14–6.98 (m, 3H), 6.72 (dd, J = 3.1 Hz, 1H), δ 2.82 (dd, J = 8.7 Hz, 2H), 1.71 (dq, J = 7.5 Hz, 2H), 1.45–1.32 (m, 7H), 0.92 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 165.49, 147.57, 140.33, 136.74, 135.35, 134.45, 127.85, 125.77, 124.84, 124.02, 123.55, 123.37; 19F NMR (400 MHz, CDCl3) δ: −135.33, −147.23, −159.49; MS m/z calculated for C28H21F5N2OS2 560.60, found 560 (M+); anal. calcd (%) for C28H21F5N2OS2: C 59.99, H 3.78, N 5.00. Found: C 59.91, H 3.69, N 4.96.
2-(4-(5-(5-hexylthiophen-2-yl)thiophen-2-yl)phenyl)-5-(naphthalen-2-yl)-1,3,4-oxadiazole (2e). Yield: 85%, colour: light brown. MP = 238–240 °C. 1H NMR (500 MHz, CDCl3) δ: 9.34 (d, J = 8.5 Hz, 1H), 8.26 (d, J = 7.0 Hz, 1H), 8.14 (d, J = 7.8 Hz, 2H), 8.04 (d, J = 8.0 Hz, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.72 (t, J = 9.2 Hz, 3H), 7.61 (t, J = 7.4 Hz, 2H), 7.31 (d, J = 2.3 Hz, 1H), 7.06 (dd, J = 2.0 Hz, 2H), 6.71 (s, 1H), δ 2.81 (t, J = 7.4 Hz, 2H), 1.70 (dd, J = 7.2 Hz, 2H), 1.38 (m, 6H), 0.94 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 164.47, 163.81, 146.07, 140.59, 138.85, 137.30, 134.36, 133.85, 132.57, 130.09, 128.69, 128.33, 128.18, 127.53, 126.71, 126.31, 125.63, 125.05, 124.92, 124.87, 123.97, 123.76, 122.22, 120.45; MS m/z calculated for C32H28N2OS2 520.71, found 520 (M+); anal. calcd (%) for C32H28N2OS2: C 73.81, H 5.42, N 5.38. Found: C 73.75, H 5.38, N 5.36.
Acknowledgements
The authors thank University Grants Commission (UGC), New Delhi, India, for financial support CPEPA No. 8-2/2008(NS/PE) and also to the University Scientific Instruments Centre, Karnatak University, Dharwad for providing the spectral data.
References
- S. J. Kim, Y. Zhang, C. Zuniga, S. Barlow, S. R. Marder and B. Kippelen, Org. Electron., 2011, 12, 492–496 CrossRef CAS PubMed.
- Y. Yang, P. Cohn, S. H. Eom, K. A. Abboud, R. K. Castellano and J. Xue, J. Mater. Chem. C, 2013, 1, 2867–2874 RSC.
- Z. Li and H. Meng, Organic Light-Emitting Materials and Devices, CRC, Boca Raton, FL, 2006 Search PubMed.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS PubMed.
- S. H. Eom, Y. Zheng, E. Wrzesniewski, J. Lee, N. Chopra, F. So and J. Xue, Appl. Phys. Lett., 2009, 94, 153303 CrossRef PubMed.
- C. Liu, Y. Li, Y. Li, C. Yang, H. Wu, J. Qin and Y. Cao, Chem. Mater., 2013, 25, 3320–3327 CrossRef CAS.
- J. Shinar and R. Shinar, J. Phys. D: Appl. Phys., 2008, 41, 133001 CrossRef.
- S. Landgraf, J. Biochem. Biophys. Methods, 2004, 61, 125–134 CrossRef CAS PubMed.
- H. van Santen and J. H. M. Neijzen, Jpn. J. Appl. Phys., 2003, 42, 1110–1112 CrossRef CAS.
- Y. Fukuda, T. Watanabe, T. Wakimoto, S. Miyaguchi and M. Tsuchida, Synth. Met., 2000, 111, 1–6 CrossRef.
- A. Haldi, J. B. Kim, B. Domercq, A. P. Kulkarni, S. Barlow, A. P. Gifford, S. A. Jenekhe, S. R. Marder and B. Kippelen, J. Disp. Technol., 2009, 5, 120–125 CrossRef CAS.
- M. K. Huda and S. K. Dolui, J. Lumin., 2010, 130, 2242–2246 CrossRef CAS PubMed.
- V. M. Niemi, P. Knuuttila, J. E. Osterholm and J. Korvola, Polymer, 1992, 33, 1559 CrossRef CAS.
- O. Bolton and J. Kim, J. Mater. Chem., 2007, 17, 1981 RSC.
- K. Lee, J. M. Rouillard, T. Pham, E. Gulari and J. Kim, Angew. Chem., Int. Ed., 2007, 46, 4667 CrossRef CAS PubMed.
- K. Okumoto and Y. Shirota, Chem. Mater., 2003, 15, 699–707 CrossRef CAS.
- S. H. Cheng, S. H. Chou, W. Y. Hung, H. W. You, Y. M. Chen, A. Chaskar, Y. H. Liu and K. T. Wonga, Org. Electron., 2013, 14, 1086–1093 CrossRef CAS PubMed.
-
(a) M. Guan, Z. Chen, Z. Bian, Z. Liu, Z. Gong, W. Baik, H. Lee and C. Huang, Org. Electron., 2006, 7, 330 CrossRef CAS PubMed;
(b) Y. Tao, Q. Wang, C. Yang, C. Zhong, K. Zhang, J. Qin and D. Ma, Adv. Funct. Mater., 2010, 20, 304 CrossRef CAS PubMed;
(c) S. Gong, Y. Chen, J. Luo, C. Yang, C. Zhong, J. Qin and D. Ma, Adv. Funct. Mater., 2011, 21, 1168 CrossRef CAS PubMed.
-
(a) H. H. Chou and C. H. Cheng, Adv. Mater., 2010, 22, 2468 CrossRef CAS PubMed;
(b) C. Fan, F. Zhao, P. Gan, S. Yang, T. Liu, C. Zhong, D. Ma, J. Qin and C. Yang, Chem.–Eur. J., 2012, 18, 5510 CrossRef CAS PubMed;
(c) S. O. Jeon and J. Y. Lee, J. Mater. Chem., 2012, 22, 4233 RSC.
-
(a) H. Inomata, K. Goushi, T. Masuko, T. Konno, T. Imai, H. Sasabe, J. J. Brown and C. Adachi, Chem. Mater., 2004, 16, 1285 CrossRef CAS;
(b) H. F. Chen, S. J. Yang, Z. H. Tsai, W. Y. Hung, T. C. Wang and K. T. Wong, J. Mater. Chem., 2009, 19, 8112 RSC.
-
(a) Z. Ge, T. Hayakawa, S. Ando, M. Ueda, T. Akiike, H. Miyamoto, T. Kajita and M. Kakimoto, Adv. Funct. Mater., 2008, 18, 584 CrossRef CAS PubMed;
(b) C. H. Chen, W. S. Huang, M. Y. Lai, W. C. Tsao, J. T. Lin, Y. H. Wu, T. H. Ke, L. Y. Chen and C. C. Wu, Adv. Funct. Mater., 2009, 19, 2661 CrossRef CAS PubMed.
- J. Ma, Z. Li, Y. Zong, Y. Men and G. Xing, Tetrahedron Lett., 2013, 54, 1348–1351 CrossRef CAS PubMed.
- Y. Liu, K. Wang, G. Huang, C. Zhu, E. Tok, K. G. Neoh and E. T. Kang, Chem. Mater., 2009, 21, 3391–3399 CrossRef CAS.
-
(a) P. Venkatakrishnan, P. Natarajan, J. N. Moorthy, Z. Lin and T. J. Chow, Tetrahedron, 2012, 68, 7502–7508 CrossRef CAS PubMed.
-
(a) C. Wang, G. Y. Jung, Y. Hua, C. Pearson, M. R. Bryce, M. C. Petty, A. S. Batsanov, A. E. Goeta and J. A. K. Howard, Chem. Mater., 2001, 13, 1167–1173 CrossRef CAS;
(b) M. Ichikawa, T. Kawaguchi, K. Kobayashi, T. Miki, K. Furukawa, T. Koyama and Y. Taniguchi, J. Mater. Chem., 2006, 16, 221 RSC;
(c) A. P. Kulkarni, C. J. Tonzola, A. Babel and S. A. Jenekhe, Chem. Mater., 2004, 16, 4556 CrossRef CAS;
(d) G. Hughes and M. R. Bryce, J. Mater. Chem., 2005, 15, 94 RSC;
(e) Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953 CrossRef CAS PubMed.
-
(a) U. Mitschke and P. Baeuerle, J. Mater. Chem., 2000, 10, 1471 RSC;
(b) P. Strohriegl and J. V. Grazulevicius, Adv. Mater., 2002, 14, 1439 CrossRef CAS.
- G. J. Zhao, R. K. Chen, M. T. Sun, J. Y. Liu, G. Y. Li, Y. L. Gao, K. L. Han, X. C. Yang and L. Sun, Chem.–Eur. J., 2006, 12, 225 CrossRef PubMed.
- A. S. Kostyuchenko, V. L. Yurpalov, A. Kurowska, W. Domagala, A. Pron and A. S. Fisyuk, Beilstein J. Org. Chem., 2014, 10, 1596–1602 CrossRef PubMed.
- M. Shailaja, M. Anitha, A. Manjula and B. Vittal Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2010, 49, 1088–1097 Search PubMed.
-
(a) K. T. Kamtekar, C. Wang, S. Bettington, A. S. Batsanov, I. F. Perepichka, M. R. Bryce, J. H. Ahn, M. Rabinal and M. C. Petty, J. Mater. Chem., 2006, 16, 3823–3835 RSC;
(b) C. Wang, L. O. Pålsson, A. S. Batsanov and M. R. Bryce, J. Am. Chem. Soc., 2006, 128, 3789–3799 CrossRef CAS PubMed;
(c) G. Mallesham, S. Balaiah, M. Ananth Reddy, B. Sridhar, P. Singh, R. Srivastava, K. Bhanuprakash and V. J. Rao, Photochem. Photobiol. Sci., 2014, 13, 342–357 RSC.
- D. J. Williams, M. W. Dimmic, W. P. Haakenson Jr, A. Wideman, B. J. Shortt, T. Cheeseright and M. J. Crawford, US Pat., US2009048311, 2009.
- S. Hou and W. K. Chan, Macromolecules, 2002, 35, 850–856 CrossRef CAS.
- S. Singh, L. K. Sharma, A. Saraswat, I. R. Siddiqui, H. K. Kehri and R. K. Pal Singh, RSC Adv., 2013, 3, 4237–4245 RSC.
- M. A. Shivkumar, K. S. Adarsh and S. R. Inamdar, J. Lumin., 2013, 143, 680–686 CrossRef CAS PubMed.
- G. Jones, W. R. Jackson and C. Choi, J. Phys. Chem., 1985, 89, 294–300 CrossRef CAS.
- D. R. T. Zahn, G. N. Gavrila and M. Gorgoi, Chem. Phys., 2006, 325, 99–112 CrossRef CAS PubMed.
- P. I. Djurovich, E. I. Mayo, S. R. Forrest and M. E. Thompson, Org. Electron., 2009, 10, 515–520 CrossRef CAS PubMed.
- S. K. Patil, M. N. Wari, C. Y. Panicker and S. R. Inamdar, Spectrochim. Acta, Part A, 2014, 123, 117–126 CrossRef CAS PubMed.
- N. Prachumrak, S. Pansay, S. Namuangruk, T. Kaewin, S. Jungsuttiwong, T. Sudyoadsuk and V. Promarak, Eur. J. Org. Chem., 2013, 6619–6628 CrossRef CAS PubMed.
- P. Kotchapadist, N. Prachumrak, T. Sunonnam, S. Namuangruk, T. Sudyoadsuk, T. Keawin, S. Jungsuttiwong and V. Promarak, Eur. J. Org. Chem., 2015, 496–505 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013 Search PubMed.
- J. Huang, Y. Jiang, J. Yang, R. Tang, N. Xie, Q. Li, H. S. Kwok, B. Z. Tang and Z. Li, J. Mater. Chem. C, 2014, 2, 2028–2036 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14550c |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (eV)
b (eV)







