Template free synthesis of graphitic carbon nitride/titania mesoflowers†
Abstract
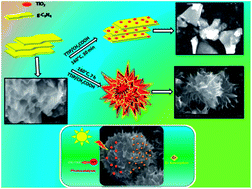
An efficient solar active catalyst is in immense demand for water splitting, waste water treatment and environmental remediation applications due to the growing energy crisis. The key to improving the activity of these materials is to increase the surface area, use a dopant and reduce the charge carrier recombination. This growing concern can be addressed by graphitic carbon nitride (g-C3N4) which has excellent visible light activity. In the present work a mesoporous flower like arrangement of carbon nitride–titania nanocomposites obtained through a solvothermal method without the use of any templates at ambient temperature is reported. A detailed investigation on the formation of these mesoflowers has been studied. The properties of the nanocomposite such as efficient absorption in the visible regime and delayed recombination of charge carriers make it an apt material for solar light photocatalysis for the degradation of organic contaminants. The high surface area of 147 m2 g−1 of the mesoflowers has been utilized for heavy metal removal such as Cr(VI) with a sorption percentage of 81% within 2 minutes of contact time in simulated water samples. This report paves the way for a new class of mesoflower nanocomposites for various environmental applications.

 Please wait while we load your content...
Please wait while we load your content...