Trimethyl-phosphite dissociative adsorption on iron by combined first-principle calculations and XPS experiments
Abstract
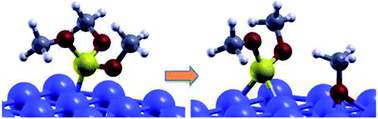
The reaction of trimethyl-phosphite, TMPi, with a clean Fe(110) surface has been investigated by ab initio calculations. The most stable configurations and energies are identified for both molecular and dissociative adsorption. The calculated reaction energies indicate that dissociation is energetically more favorable than molecular adsorption and we provide a description of the dissociation path and the associated energy barrier. In situ XPS analysis of adsorbed TMPi on metallic iron confirmed molecular chemisorption and dissociation at high temperature. These results shed light on the mechanism of phosphorus release from organophosphites at the iron surface, which is important for the functionality of these phosphorus-based additives, included in lubricants for automotive applications.

 Please wait while we load your content...
Please wait while we load your content...