Efficient and rapid direct transesterification reactions of cellulose with isopropenyl acetate in ionic liquids†
Abstract
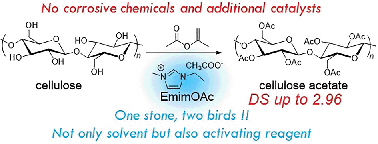
We describe a conceptually novel protocol that allowed the facile transesterification reaction of cellulose without any additional catalysts and corrosive chemicals. The key concept in this cellulose modification is the dual functionalities of ionic liquids, namely a solvent for reactants and an activating reagent for transesterification reactions.

 Please wait while we load your content...
Please wait while we load your content...