Synthesis, photophysical and electrochemical properties of a new class of fluorescent amidoanthracenophanes†
Abstract
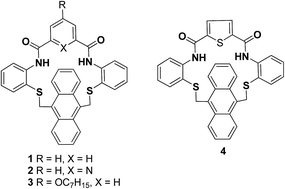
Fluorescent amidoanthracenophanes 1, 2, 3 and 4 were obtained from the various aromatic diacid chlorides and diamino precyclophane by simple acylation. All the synthesized amidoanthracenophanes 1, 2, 3 and 4 show three intense fluorescence bands between 401 and 402, 426 and 429, and at 449 to 451 nm, and exhibit two/three oxidation reduction peaks in the cyclic voltammetry which is characteristic of fluorophoric anthracene units.

 Please wait while we load your content...
Please wait while we load your content...