Identification and characterization of a cold-adapted and halotolerant nitrobenzene-degrading bacterium
Xiping Maa,
Xianzhu Lia,
Wanlong Lia,
Di Wanga,
Chengbin Xu*a and
Xuelian Meng*b
aSchool of Environmental Science, Liaoning University, Shenyang 110036, China. E-mail: xuchengbin80@163.com; Fax: +86 24 62204818; Tel: +86 24 62204818
bSchool of Pharmacy, Liaoning University, Shenyang 110036, China. E-mail: rubymxl@163.com; Fax: +86 24 62204818; Tel: +86 24 62202248
First published on 9th September 2015
Abstract
A strain named X7, which uses nitrobenzene (NB) as a carbon source at low temperature and high salinity, was domesticated and isolated from activated sludge in the aeration tank of Shenyang Northern Sewage Treatment Plant. According to its morphological, physiological and biochemical characteristics and 16S rDNA gene sequence, a preliminary identification was made. The result showed that strain X7 was Myroides odoratus. The optimal degradation conditions of strain X7 were as follows: inoculum concentration 10%, optimal temperature 15 °C, pH 7.0, rotary speed 150 rpm and salinity 1.0%–3.0%. At an initial concentration of nitrobenzene of 150 mg L−1, the degradation rate was 51.50%. Moreover, the addition of glucose and peptone could increase the degradation rate to 58.92% and 60.24%, respectively. The maximum concentration of nitrobenzene that was tolerated by strain X7 was 350 mg L−1.
1 Introduction
Nitrobenzene (NB), which is an important chemical material, is widely used in the manufacture of organic products such as aniline, dyes, drugs, explosives, insecticides and synthetic rubber.1 Nitrobenzene has been proven to be a carcinogen and a huge threat to human health.2,3 Therefore, nitrobenzene is listed as a priority pollutant by the US Environmental Protection Agency.4Much effort has been made to treat contamination by nitrobenzene.5–8 Compared to physical and chemical methods, biological methods can mineralize nitrobenzene at a lower cost.9–11 Bacterial strains capable of degrading nitrobenzene have been described such as Bacillus cereus, Streptomyces, Rhodotorula mucilaginosa, white rot fungi, and Methanothrix soehngenii.12–17
Much research has described the effects of environmental factors on the degradation of nitrobenzene by microorganisms, but most of the degradation studies were conducted at room temperature.18,19 There have only been a few reports that studied the degradation of nitrobenzene in extreme environments.20 Therefore, it is essential to test the degradation of nitrobenzene by strains at low temperature and high salinity and evaluate their potential application in the treatment of nitrobenzene in wastewater.
In this study, a strain that uses nitrobenzene as a carbon source at low temperature and high salinity was domesticated and isolated from activated sludge in the aeration tank of Shenyang Northern Sewage Treatment Plant. The effects of several parameters, including inoculum, pH, temperature, salinity and auxiliary carbon/nitrogen sources, as co-substrates on the biodegradation of nitrobenzene by the strain were investigated.
2 Materials and methods
2.1 Bacterial sources
The source of the strain used in this study was obtained from activated sludge in the aeration tank of Shenyang Northern Sewage Treatment Plant.2.2 Medium
Enriched medium contained peptone 10 g L−1, beef extract 3 g L−1, and NaCl 5 g L−1 at pH 7.0. Mineral salt medium contained Na2HPO4·12H2O 3.8 g L−1, KH2PO4 1 g L−1, NaCl 1 g L−1, MgSO4 0.2 g L−1, and NH4Cl 0.1 g L−1. Isolation medium contained peptone 10 g L−1, beef extract 3 g L−1, NaCl 30 g L−1, and agar gel 15%–20% at pH 7.0.2.3 Analytical methods
Nitrobenzene concentrations were measured with a Cary 50 UV-vis spectrophotometer (Varian, US) using N-(1-naphthyl)-ethylenediamine dihydrochloride as per standard procedure. Bacterial cell concentrations were determined by a photoelectric turbidimetry method. A UV-vis spectrophotometer was used to determine the growth of bacteria by monitoring the optical density at a wavelength of 600 nm (OD600).2.4 Acclimation, screening and separation of strains
To obtain strains that were tolerant of nitrobenzene, 10 mL activated sludge was first inoculated into 90 mL enriched medium in a 250 mL flask containing 10 mg L−1 nitrobenzene. When the culture became turbid, 10 mL of the culture was transferred to 90 mL fresh MSM in a new 250 mL flask containing 10 mg L−1 nitrobenzene. The 250 mL flask was supplemented with nitrobenzene to a final concentration of 150 mg L−1 and cultivated on a rotary shaker (150 rpm) at 30 °C. When the degradation rate of nitrobenzene reached a stable level, the activated sludge was domesticated at low temperature and high salinity. When the temperature decreased to 15 °C and the salinity of the medium increased to 3.0%, the culture was diluted and spread onto agar plates with isolation medium at 150 mg L−1. The agar plates were incubated at 15 °C for 5–7 days. Colonies that appeared on the agar plates were subcultured. Five species of strains with nitrobenzene as a carbon source were screened after microscopic examination. After a degradation process, a strain that provided better degradation of nitrobenzene, named X7, was screened.2.5 Strain identification
According to the strain's Gram stain, individual form, and colony, physiological and biochemical characteristics, a preliminary identification was made.With the strain's DNA as a template, extracted DNA from the target strain X7 and a partial 16S rDNA sequence were amplified by PCR using the following primers: F27 (5′-AGACTTTGATCCTGGCTCAG-3′) as forward and R1492 (5′-ACGGTTACCTGTTACGACTT-3′) as reverse. The system contained 1 μL DNA template, 2 μL dNTP, 2.5 μL 10 × PCR buffer, 1 μL of each primer, 0.2 μL Taq polymerase, and 17.3 μL deionized water. PCR reactions were performed under the following conditions: 5 min at 94 °C; 30 cycles of 30 s at 94 °C, 30 s at 58 °C, and 90 s at 72 °C; plus an additional 10 min cycle at 72 °C. PCR fragments were ligated into the linear vector pMD18-T (TaKaRa Biotechnology, Dalian Co., Ltd, China) after purification by agarose gel electrophoresis and then transformed into competent Escherichia coli DH5α cells. The 16S rDNA gene sequence was completed by Harbin Boshi Biological Technology Co. Ltd. The partial 16S rDNA sequence has been submitted to GenBank, and BioEdit v 5.06 was used to analyze multiple sequence alignments. The sequences were analyzed by MEGA 6.0. A phylogenetic tree was obtained by a neighbor-joining method.
2.6 Growth curve of strain X7
Ten milliliters of the culture in the late exponential phase was aseptically inoculated into each 250 mL flask with 90 mL sterilized MS medium. The 250 mL flasks were supplemented with nitrobenzene (150 mg L−1) and cultivated on a rotary shaker (150 rpm) at 15 °C. Samples were withdrawn from each flask at 1 day intervals and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was diluted and used to determine the nitrobenzene concentration. Three parallel samples were taken for each experiment. All experiments were performed in triplicate.
000 rpm for 10 min. The supernatant was diluted and used to determine the nitrobenzene concentration. Three parallel samples were taken for each experiment. All experiments were performed in triplicate.
2.7 Effect of several parameters on the growth and degradation rate of X7
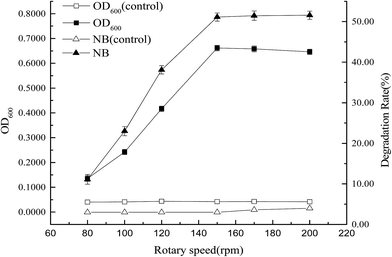
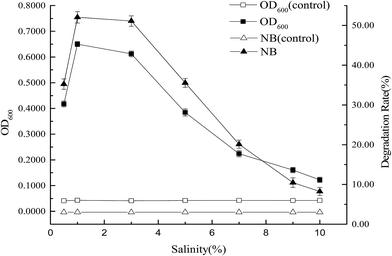
The effects of parameters, such as inoculum concentration (1%, 3%, 5%, 10%, 15%, and 20%), pH (4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0), rotary speed (80, 100, 120, 150, 170, and 200 rpm), temperature (5, 10, 15, 20, 25, and 30 °C), and salinity [0.5%, 1.0%, 3.0%, 5.0%, 7.0%, 9.0%, and 10.0% NaCl (w/v)] on the degradation of nitrobenzene by strain X7, were investigated. The initial concentration of nitrobenzene was 150 mg L−1. Samples were withdrawn from each flask at 7 d and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was diluted and used to determine the nitrobenzene concentration. Non-inoculated cultures with the same concentrations of nitrobenzene served as controls. Three parallel samples were taken for each experiment. All experiments were performed in triplicate.
000 rpm for 10 min. The supernatant was diluted and used to determine the nitrobenzene concentration. Non-inoculated cultures with the same concentrations of nitrobenzene served as controls. Three parallel samples were taken for each experiment. All experiments were performed in triplicate.
2.8 Effect of co-substrate on the growth and degradation rate of X7
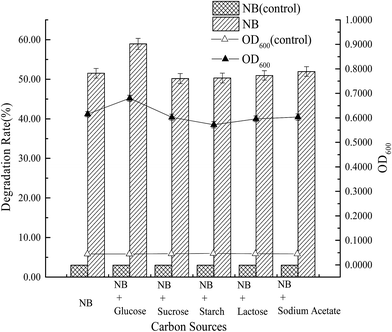
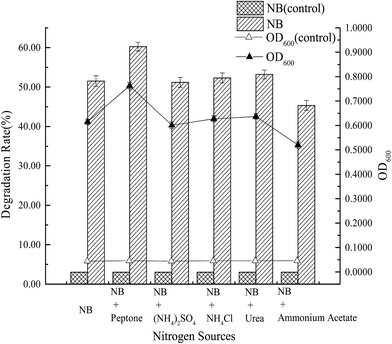
The effects of co-substrates, such as a carbon source (1% glucose, 1% sucrose, 1% starch, 1% lactose, 1% sodium acetate) and a nitrogen source (1% peptone, 1% ammonium sulfate, 1% ammonium chloride, 1% urea, 1% ammonium acetate) on the degradation of nitrobenzene by strain X7, were also investigated. A 250 mL flask was supplemented with nitrobenzene (150 mg L−1) and cultivated on a rotary shaker (150 rpm) at 15 °C. Samples were withdrawn from each flask at 7 d and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was diluted and used to determine the nitrobenzene concentration. Three parallel samples were taken for each experiment. All experiments were performed in triplicate.
000 rpm for 10 min. The supernatant was diluted and used to determine the nitrobenzene concentration. Three parallel samples were taken for each experiment. All experiments were performed in triplicate.
2.9 Test of the tolerance of strain X7 for nitrobenzene
One milliliter of culture in the late exponential phase was inoculated onto MSM agar plates with increasing concentrations of nitrobenzene. The plates were incubated at 15 °C for a week and the tolerance level of the culture to nitrobenzene was determined.3 Results
3.1 Strain screening
At low temperature and high salinity, sixteen strains that use nitrobenzene as a carbon source were domesticated and isolated from activated sludge in the aeration tank of Shenyang Northern Sewage Treatment Plant. The strains were cultivated with MS medium under conditions of rotary speed 150 rpm, salinity 3.0%, and temperature 15 °C. The concentration of nitrobenzene was 150 mg L−1. Nitrobenzene degradation rates of sixteen strains are shown in Fig. 1. Five (X1, X3, X7, X11 and X14) of them could grow well and their degradation rates of nitrobenzene were all over 40%. Then, those five strains were cultivated with MS medium under conditions of rotary speed 150 rpm, salinity 3.0%, and temperature 15 °C. Nitrobenzene degradation rates of five strains are shown in Fig. 2. The degradation rate of nitrobenzene of strain X7 was very high. Therefore, strain X7 was identified as a cold-adapted and halotolerant nitrobenzene-degrading bacterium.3.2 Identification of strain X7
| Strain | Starch hydrolysis experiment | Glucose oxidation fermentation test | Lactose fermentation test | MR test | VP test | Indole test | Litmus milk test | Gelatin liquefaction test | Catalase | Citrate utilization test |
|---|---|---|---|---|---|---|---|---|---|---|
| X7 | − | − | − | − | − | − | White | + | + | − |
According to the test results, strain X7 was identified as a Flavobacterium sp. by Bergey's Manual of Determinative Bacteriology and the Manual of Systematic Methods of Determinative Bacteriology.
3.3 Growth curve of strain X7
The growth curve of strain X7 and its degradation rate of nitrobenzene during 10 days are shown in Fig. 4. The strain grew slowly during the first two days, which was the adaptation period. From 3 to 6 d was the logarithmic growth phase. Both the growth of the strain and the degradation rate of nitrobenzene increased rapidly. From 6 to 8 d was the stationary phase. On the seventh day, the highest degradation rate was 51.50%. The growth of strain X7 and the degradation rate of nitrobenzene were stable after the eighth day, which may be due to inhibition by metabolic intermediate products from the degradation of nitrobenzene by strain X7.19 Finally, 7 d was determined to be the best time for degradation in this study.3.4 Optimal degradation conditions of X7
| Strain | Concentration of nitrobenzene (mg L−1) | ||||||
|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | 250 | 300 | 350 | 400 | |
| X7 | + | + | + | + | ± | ± | − |
4 Conclusions
(1) Five (X1, X3, X7, X11 and X14) strains that use nitrobenzene as a carbon source were domesticated and isolated from activated sludge in the aeration tank of Shenyang Northern Sewage Treatment Plant. At an initial concentration of nitrobenzene of 150 mg L−1, the degradation rate of nitrobenzene was over 40%. The nitrobenzene degradation rate of strain X7 was uniformly high. Therefore, strain X7 was identified as a cold-adapted and halotolerant nitrobenzene-degrading bacterium.(2) According to strain X7's morphological, physiological and biochemical characteristics and 16S rDNA gene sequence, the result showed that strain X7 was Myroides odoratus.
(3) From tests on the growth and degradation processes of strain X7, the optimal degradation conditions of strain X7 were as follows: inoculum concentration 10%, optimal temperature 15 °C, pH 7.0, rotary speed 150 rpm and salinity 1.0%–3.0%. At an initial concentration of nitrobenzene of 150 mg L−1, the degradation rate was 51.50%. However, the addition of glucose and peptone could increase the degradation rate to 58.92% and 60.24%, respectively. The maximum tolerated concentration of nitrobenzene of strain X7 was 350 mg L−1.
Acknowledgements
This study was supported by National “12·5” water special sub-topics (2012ZX07202003-05); Funded projects of the 211 key discipline of Environmental Institute, Liaoning University; Research of teaching reform project of undergraduate, Liaoning University (No. JG2013ZD0010).References
- J. Ye, A. Singh and O. P. Ward, Microb. Biotechnol., 2004, 20, 117–135 CrossRef CAS.
- H. L. Li, Y. Cheng, H. F. Wang, H. F. Sun, Y. F. Liu, K. X. Liu and S. X. Peng, Appl. Radiat. Isot., 2003, 58, 1131–1142 CrossRef.
- Y. L. Zhang, K. Zhang, C. M. Dai and X. F. Zhou, Chem. Eng. Sci., 2014, 111, 135–141 CrossRef CAS PubMed.
- Y. Z. Lin, J. Yin, J. H. Wang and W. D. Tian, Bioresour. Technol., 2012, 118, 128–135 CrossRef CAS PubMed.
- Y. X. Yang, J. Ma, Q. D. Qin and X. D. Zhai, J. Mol. Catal. A: Chem., 2007, 267, 41–48 CrossRef CAS PubMed.
- S. J. Zhang, H. Jiang, M. J. Li, H. Q. Yu, H. Yin and Q. R. Li, Environ. Sci. Technol., 2007, 41, 1977–1982 CrossRef CAS.
- Q. Wen, Z. Chen, J. Lian, Y. Feng and N. Ren, J. Hazard. Mater., 2012, 209–210, 226–232 CrossRef CAS PubMed.
- B. W. Zhou, J. L. Song, H. C. Zhou, L. Q. Wu, T. B. Wu, Z. M. Liu and B. X. Han, RSC Adv., 2015, 5, 36347–36352 RSC.
- D. F. Zhao, C. S. Liu, Y. B. Zhang and Q. Y. Liu, Desalination, 2011, 281, 17–22 CrossRef CAS PubMed.
- T. Li, X. P. Deng, J. J. Wang, Y. C. Chen, L. He, Y. C. Sun, C. X. Song and Z. F. Zhou, Mar. Pollut. Bull., 2014, 89, 384–389 CrossRef CAS PubMed.
- Q. X. Zhou, C. Y. Diao, Y. B. Sun and J. L. Zhou, Chemosphere, 2012, 86, 994–1000 CrossRef CAS PubMed.
- C. B. Xu, Y. G. Wang and X. P. Ma, Acta Sci. Circumstantiae, 2012, 32, 1326–1332 CAS.
- C. L. Zheng, J. T. Zhou, L. H. Zhao, H. Lu, B. C. Qu and J. Wang, Bull. Environ. Contam. Toxicol., 2007, 78, 153–157 CrossRef PubMed.
- C. L. Zheng, J. T. Zhou, J. Wang, J. Wang and B. C. Qu, J. Hazard. Mater., 2008, 160, 194–199 CrossRef CAS PubMed.
- L. Levin, A. Viale and A. Forchiassin, Int. Biodeterior. Biodegrad., 2003, 52, 1–5 CrossRef CAS.
- Y. Z. Lin, X. K. Han, H. Lu and J. L. Zhou, Int. Biodeterior. Biodegrad., 2013, 85, 499–505 CrossRef CAS PubMed.
- C. L. Zheng, J. T. Zhou, J. Wang, B. C. Qu, J. Wang, H. Lu and H. X. Zhao, J. Hazard. Mater., 2009, 100, 2082–2084 CAS.
- D. F. Zhao, C. S. Liu, Y. B. Zhang and Q. Y. Liu, Desalination, 2011, 281, 17–22 CrossRef CAS PubMed.
- C. L. Zheng, J. T. Zhou, J. Wang, B. C. Qu, J. Wang, H. Lu and H. X. Zhao, Bioresour. Technol., 2009, 100(6), 2082–2084 CrossRef CAS PubMed.
- N. Liu, H. J. Li, F. Ding, Z. M. Xiu, P. Liu and Y. Yu., J. Hazard. Mater., 2013, 260, 323–329 CrossRef CAS PubMed.
- S. Chatterjee, S. K. Das, R. Chakravarty, A. Chakrabarti, S. Ghosh and A. K. Guha, J. Hazard. Mater., 2010, 174, 47–53 CrossRef CAS PubMed.
- D. Z. Wang, G. Y. Zheng, S. M. Wang, D. W. Zhang and L. X. Zhou, J. Environ. Sci., 2011, 23(12), 2063–2068 CrossRef CAS.
- S. K. Das, I. Shome and A. K. Guha, Sep. Sci. Technol., 2012, 47, 1913–1925 CrossRef CAS PubMed.
- Z. L. Li, M. Yang, D. Li, R. Qi, H. J. Liu, J. F. Sun and J. H. Qu, J. Environ. Sci., 2008, 20(7), 778–786 CrossRef CAS.
- J. L. Chen, M. H. Wong, Y. S. Wong and N. F. Y. Tam, Mar. Pollut. Bull., 2008, 57, 695–702 CrossRef CAS PubMed.
- T. Li, X. P. Deng, J. J. Wang, Y. C. Chen, L. He, Y. C. Sun, C. X. Song and Z. F. Zhou, Mar. Pollut. Bull., 2014, 89, 384–389 CrossRef CAS PubMed.
- D. Z. Wang, G. Y. Zheng and L. X. Zhou, Bioresour. Technol., 2012, 110, 91–96 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |