Tetramethylguanidium-based ionic liquids as efficient and reusable catalysts for the synthesis of biscoumarin at room temperature†
Anlian Zhu,
Mingyue Wang,
Lingjun Li and
Jianji Wang*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: jwang@htu.cn; Fax: +86-0373-3329030; Tel: +86-0373-3325805
First published on 20th August 2015
Abstract
In this work, a basic ionic liquid, tetramethylguanidium acetate ([TMG][Ac]), was found to be an efficient catalyst for the one-pot preparation of biscoumarins through a domino Knoevenagel–Michael reaction at room temperature. This novel catalytic system avoids the utilization of volatile organic solvents and has excellent tolerance to various functional groups on substrates. The convenient preparation, excellent catalytic efficiency, good reusability and the perspective in scale-up reactions make this catalyst intriguing in industrial applications.
Introduction
In the past decades, ionic liquids (ILs) have been widely utilized in catalysis and organic synthesis as substitutes for volatile organic solvents mainly due to their excellent solubility and low vapor pressure.1–3 However, with the development of task-specific ionic liquids, the functions of ionic liquids have been extended to catalysts, extractants, absorbents, structure orientation regents, etc.4–8 Although the research and development of basic ionic liquids is relatively unparalleled compared with acidic ionic liquids, promising perspectives have been shown for such a class of ionic liquids.9 For example, amine-functionalized imidazolium ionic liquids were found to be efficient catalysts for the hydrogenation of carbon dioxide.10,11 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) or 1,4-diazabicyclo[2.2.2]octane (DABCO)-based basic ionic liquids also showed excellent catalytic activities for the fixation of CO2 (ref. 12–14) and various multi-component reactions.15,16 In our previous work, a series of basic ionic liquids based on cholinium or guanidium were synthesized and utilized in aldol and Knoevenagel–Henry reactions, and a difference in chemo- or regioselectivity was found between these basic catalytic systems and traditional acidic catalysis systems.17–20Biscoumarins are an important kind of vitamin K antagonists which have wide biological activities such as anti-HIV, anti-bacterial, anti-oxidant, anti-cancer, and anti-coagulant activities.21–24 Recently, it was found that the inhibition of NAD(P)H:quinone oxidoreductase (NQO1) with biscoumarins can induce cell killing and oxidative stress in pancreatic cancer cells.25 Several methods have been adopted for the synthesis of biscoumarins through the catalyzed domino Knoevenagel–Michael reactions of aldehydes with two equivalents of 4-hydroxycoumarin. Actually, this domino reaction can also be catalyzed by acidic catalysts such as molecular iodine,26 Zn (proline)2,27 sodium dodecyl sulfate,28 heteropoly acids,29 nano silica chloride,30 propane-1,2,3-triyltris(hydrogen sulfate),31 and SO3H-functionalized ionic liquids.32,33 However, these catalytic systems often suffer from high reaction temperature, low yield, long reaction time and necessary refluxing in volatile organic solvents. Recently, basic ionic liquids based on N-hydroxyethylammonium type cations were found to catalyze this reaction at relatively mild conditions but still suffered from low or moderate isolated yields for the target compounds at room temperature.34,35 Therefore, it is still interesting to develop a reusable catalytic system which can efficiently catalyze these types of reactions at room temperature from the viewpoint of atom economy and energy efficiency.
Herein, the catalytic performances of a series of basic tetramethylguanidium (TMG)-based ionic liquids in domino Knoevenagel–Michael reactions have been investigated for the one-pot synthesis of biscoumarins. It is shown that tetramethylguanidium acetate has the highest catalytic activity for the domino Knoevenagel–Michael reaction, and the target biscoumarins with various functional groups can be synthesized with excellent isolated yields at room temperature. In addition, the catalyst is easy to regenerate and reuse, and the catalytic activity has no significant decrease after six runs.
Results and discussion
The catalytic performances of tetramethylguanidium-based ionic liquids with different anions in one-pot Knoevenagel–Michael reactions were investigated by using the reaction between benzaldehyde and 4-hydroxycoumarin as a model. The influence of the reaction temperature and the amounts of the ILs on the catalytic performance were also studied. It is clear from Table 1 that the ionic liquids with acetate (Ac), propionate (Pr) and lactate (Lac) as anions could catalyze this reaction. Nevertheless, the ionic liquid with a TMG cation and an Ac anion has the highest catalytic activity, and the target compound can arrive at 96% isolated yield at room temperature within 3.5 hours when the molar ratio of the ionic liquid to the aldehyde was increased to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| Entry | IL | Aldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) coumarin coumarin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL molar ratio IL molar ratio |
Temperature (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yields. | |||||
| 1 | [TMG][Ac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
50 | 2 | 64 |
| 2 | [TMG][Pr] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
50 | 4 | 49 |
| 3 | [TMG][Lac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
50 | 4 | 51 |
| 4 | [TMG][Ac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
25 | 2 | 53 |
| 5 | [TMG][Ac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
25 | 3 | 64 |
| 6 | [TMG][Pr] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
25 | 3 | 61 |
| 7 | [TMG][Lac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
25 | 4 | 60 |
| 8 | [TMG][Lac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
25 | 2 | 73 |
| 9 | [TMG][Ac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
25 | 4 | 90 |
| 10 | [TMG][Ac] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
25 | 3.5 | 96 |
In order to evaluate the generality of this catalyst, a range of biscoumarin derivatives were prepared under the optimized reaction conditions using the reactions of 4-hydroxycoumarin with various aldehydes. The results were collected in Table 2. It can be seen that in all cases, aromatic aldehydes with substituents bearing either electron donating or withdrawing groups reacted smoothly and gave the target biscoumarin products with excellent isolated yields (entries 1–13). Heteroaromatic aldehydes such as 4-pyridinecarboxaldehyde and 2-furaldehyde could also react with 4-hydroxycoumarin efficiently to give excellent yields for the target compounds (entries 14 and 15). Piperonyl aldehyde with acid sensitive groups on the aromatic ring also showed excellent reactivity under the catalysis of [TMG][Ac], leaving the acetal group intact (entry 16). The structures of all these products were confirmed using 1H NMR spectral data, and were found to be in accordance with those reported in the literature.26–34
| Entry | Aldehyde | Product | Time (h) | Yielda,b (%) |
|---|---|---|---|---|
| a Reaction conditions: aldehyde (0.5 mmol), 4-hydroxycoumarin (1 mmol), tetramethyl guanidine acetate (0.75 mmol), room temperature.b Isolated yields. | ||||
| 1 |  |
 |
3.5 | 96 |
| 2 |  |
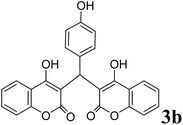 |
3.5 | 92 |
| 3 | 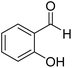 |
 |
0.5 | 90 |
| 4 |  |
 |
0.5 | 93 |
| 5 |  |
 |
1.5 | 88 |
| 6 |  |
 |
4 | 96 |
| 7 |  |
 |
4 | 91 |
| 8 |  |
 |
2.5 | 90 |
| 9 | 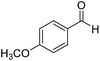 |
 |
4.5 | 86 |
| 10 |  |
 |
2 | 99 |
| 11 |  |
 |
2 | 87 |
| 12 |  |
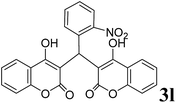 |
3 | 92 |
| 13 |  |
 |
2 | 84 |
| 14 |  |
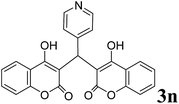 |
4 | 87 |
| 15 |  |
 |
0.5 | 95 |
| 16 |  |
 |
2.5 | 98 |
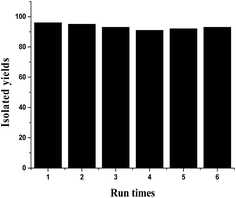
From an industrial point of view, the scale-up reaction and reusability of the catalyst are two important aspects for the application of a given catalytic system. The scale-up reaction between benzaldehyde (10 mmol) and 4-hydroxylcoumarin (20 mmol) was conducted under the optimized conditions. The result showed that an almost quantitative yield of the target compound was achieved, suggesting that this catalytic system is easy to scale up. The reusability of the ionic liquid was also examined using the reaction between 4-hydroxylcoumarin and 4-nitroaldehyde. The ionic liquid can be recovered from the reaction system using water as the extracting agent, and then reused directly after drying under vacuum. The isolated yields for the target compound in different cycles are illustrated in Fig. 1. It is clear that after six cycles, the catalytic activity of the catalyst has no significant decrease. Therefore, it can be concluded that this catalytic system has great potential in industrial applications.
In addition, a plausible mechanism for the [TMG][Ac]-catalyzed one-pot Knoevenagel–Michael reaction is outlined in Scheme 1. As a hydrogen bond donor, NH2 groups on the cation of the IL activated the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the aldehyde, and the Ac anion of the IL activated the O–H of 4-hydroxylcoumarin as a hydrogen bond acceptor. This dual activation effect from the ionic liquid led to the addition of one molecule of 4-hydroxylcoumarin to one aldehyde molecule. Then, under the catalysis of the basic Ac anion, intermediate 4 was dehydrated to produce intermediate 5, which was also activated by the cation of the ionic liquid and then reacted with another molecule of 4-hydroxylcoumarin activated by the anion of the ionic liquid, leading to the target compound with high efficiency. In this catalytic procedure, the cation and the anion have a synergetic effect on the substrates. This is a possible reason why this catalytic system could reduce the reaction temperature significantly.
O of the aldehyde, and the Ac anion of the IL activated the O–H of 4-hydroxylcoumarin as a hydrogen bond acceptor. This dual activation effect from the ionic liquid led to the addition of one molecule of 4-hydroxylcoumarin to one aldehyde molecule. Then, under the catalysis of the basic Ac anion, intermediate 4 was dehydrated to produce intermediate 5, which was also activated by the cation of the ionic liquid and then reacted with another molecule of 4-hydroxylcoumarin activated by the anion of the ionic liquid, leading to the target compound with high efficiency. In this catalytic procedure, the cation and the anion have a synergetic effect on the substrates. This is a possible reason why this catalytic system could reduce the reaction temperature significantly.
Conclusion
A sustainable catalytic system for the one-pot preparation of biscoumarins was developed through a domino Knoevenagel–Michael reaction at room temperature. The [TMG][Ac] catalyst is very cheap, easy to prepare, easy to handle and has a high catalytic activity and selectivity for a wide range of substrates. The weak basic property of this catalyst leads to its excellent performance in the conversion of reactants involving acid sensitive groups. In addition, this catalyst is feasible for scale-up, which makes it promising in the industrial production of biscoumarins.Experimental
All starting materials (A. R. grade) were purchased from Beijing chemical reagents company and used without further purification. 1,1,3,3-Tetramethylguanidium-based ionic liquids were prepared by neutralizing equivalent molar TMG with the corresponding carboxyl acids according to the procedure described in the literature.17–19Typical experimental procedures for the preparation of biscoumarins using the ionic liquid catalyst
1.5 mmol of tetramethylguanidium acetate was added to the mixtures containing 1 mmol of aldehyde and 2 mmol of 4-hydroxylcoumarin under stirring at room temperature. The extent of the reaction was monitored using TLC analysis. After the reaction was completed, the reaction mixture was washed with water three times and the ionic liquid catalyst was recovered and reutilized directly from the combined water phase after water evaporation. The pure biscoumarin product can be obtained through recrystallization from an aqueous ethanol solution.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21373079) and the Program for Science & Technology Innovation Talents in Universities of Henan Province (No. 14HASTIT015).References
- J. Dupont, R. F. de Souza and P. A. Z. Auarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS PubMed.
- W. S. Miao and T. H. Chan, Acc. Chem. Res., 2006, 39, 897 CrossRef CAS PubMed.
- C. Hardacre, J. D. Holbrey, M. Nieuwenhuyzen and T. G. A. Youngs, Acc. Chem. Res., 2007, 40, 1146 CrossRef CAS PubMed.
- J. W. Lee, J. Y. Shin, Y. S. Chun, H. B. Jang, C. E. Song and S.-G. Lee, Acc. Chem. Res., 2010, 43, 985 CrossRef CAS PubMed.
- X. X. Han and D. W. Armstrong, Acc. Chem. Res., 2007, 40, 1079 CrossRef CAS PubMed.
- J. Dupont, Acc. Chem. Res., 2011, 44, 1223 CrossRef CAS PubMed.
- S. J. Zhang, J. Sun, X. C. Zhang, J. Y. Xin, Q. Q. Miao and J. J. Wang, Chem. Soc. Rev., 2014, 43, 7838 RSC.
- P. Pollet, E. A. Davey, E. E. Ureña-Benavides, C. A. Eckert and C. L. Liotta, Green Chem., 2014, 16, 1034 RSC.
- D. R. MacFarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 2006, 1905 RSC.
- Z. F. Zhang, Y. Xie, W. J. Li, S. Q. hu, J. L. Song, T. Jiang and B. X. Han, Angew. Chem., Int. Ed., 2008, 47, 1127 CrossRef CAS PubMed.
- Z. F. Zhang, S. Q. Hu, J. L. Song, W. J. Li, G. Y. Yang and B. X. Han, ChemSusChem, 2009, 2, 234 CrossRef CAS PubMed.
- W. J. Lu, J. Ma, J. Y. Hu, J. L. Song, Z. F. Zhang, G. Y. Yang and B. X. Han, Green Chem., 2014, 16, 221 RSC.
- Z.-Z. Yang, L.-N. He, C.-X. Miao and S. Chanfreau, Adv. Synth. Catal., 2010, 352, 2233 CrossRef CAS PubMed.
- Y. F. Zhao, B. Yu, Z. Z. Yang, H. Y. Zhang, L. D. Hao, X. Gao and Z. M. Liu, Angew. Chem., Int. Ed., 2014, 53, 23 Search PubMed.
- D. S. Raghuvanshi and K. N. Singh, Tetrahedron Lett., 2011, 52, 5702 CrossRef CAS PubMed.
- L. Chen, Y.-Q. Li, X.-J. Huang and W.-J. Zheng, Heteroat. Chem., 2009, 20, 91 CrossRef CAS PubMed.
- A. L. Zhu, T. Jiang, D. Wang, B. X. Han, L. Liu, J. Huang, J. C. Zhang and D. H. Sun, Green Chem., 2005, 7, 514 RSC.
- A. L. Zhu, T. Jiang, B. X. Han, J. Huang, J. C. Zhang and X. M. Ma, New J. Chem., 2006, 30, 736 RSC.
- J. C. Zhang, T. Jiang, B. X. Han, A. L. Zhu and X. M. Ma, Synth. Commun., 2006, 36, 3305 CrossRef CAS PubMed.
- A. L. Zhu, R. X. Liu, L. J. Li, L. Y. Li, L. Wang and J. J. Wang, Catal. Today, 2013, 200, 17 CrossRef CAS PubMed.
- A. Maresca, A. Scozzafava and C. T. Supuran, Bioorg. Med. Chem. Lett., 2010, 20, 7255 CrossRef CAS PubMed.
- N. Vukovic, S. Sukdolak, S. Solujic and N. Niciforovic, Food Chem., 2010, 120, 1011 CrossRef CAS PubMed.
- H. Madari, D. Panda, L. Wilson and R. Jacobs, Cancer Res., 2003, 63, 1214 CAS.
- F. Carta, A. Maresca, A. Scozzafava and C. T. Supuran, Bioorg. Med. Chem., 2012, 20, 2266 CrossRef CAS PubMed.
- A. Lewis, M. Ough, L. Li, M. M. Hinkhouse, J. M. Ritchie, D. R. Spitz and J. J. Cullen, Clin. Cancer Res., 2004, 10, 4550 CrossRef CAS PubMed.
- M. Kidwai, V. Bansal, P. Mothsra, S. Saxena, R. K. Somvanshi, S. Dey and T. P. Singh, J. Mol. Catal. A: Chem., 2007, 268, 76 CrossRef CAS PubMed.
- Z. N. Siddiqui and F. Farooq, Catal. Sci. Technol., 2011, 1, 810 Search PubMed.
- H. Mehrabi and H. Abusaidi, J. Iran. Chem. Soc., 2010, 7890 Search PubMed.
- P. Singh, P. Kumar, A. Katyal, R. Kalra, S. K. Dass, S. Prakash and R. Chandra, Catal. Lett., 2010, 134, 303 CrossRef CAS.
- R. Karimian, F. Piri, A. A. Safari and S. J. Davarpanah, J. Nano. Chem., 2013, 3, 52 CrossRef.
- R. Rezaei, F. Moezzi and M. M. Doroodmand, Chin. Chem. Lett., 2014, 184, 183 CrossRef PubMed.
- W. Li, Y. Wang, Z. Z. Wang, L. Y. Dai and Y. Y. Wang, Catal. Lett., 2011, 141, 1651 CrossRef CAS.
- K. P. Boroujeni and P. Ghasemi, Catal. Commun., 2013, 37, 50 CrossRef PubMed.
- A. Tzani, A. Douka, A. Papadopoulos, E. A. Pavlatou, E. Voutsas and A. Detsi, ACS Sustainable Chem. Eng., 2013, 1, 1180 CrossRef CAS.
- A. L. Zhu, S. K. Bai, L. J. Li, M. Y. Wang and J. J. Wang, Catal. Lett., 2015, 145, 1089 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14247d |
| This journal is © The Royal Society of Chemistry 2015 |