Endophyte inspired chemical diversity from beta-caryophyllene†
Hao-Yu Tanga,
Jin-Ming Gao*a and
Qiang Zhang*ab
aShaanxi Key Laboratory of Natural Products & Chemical Biology, College of Science, Northwest A&F University, Yangling, 712100, China. E-mail: jinminggao@nwsuaf.edu.cn; zerkang@126.com
bState Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin, 300071, China
First published on 19th August 2015
Abstract
The natural product (−)-β-caryophyllene is considered as an ideal initiator to generate diverse scaffolds by transannular cyclization due to its macrocyclic structure and abundant availability in nature. An endophytic strain Aspergillus tubingensis KJ-9 was screened in our lab to catalyse remodeling of the β-caryophyllene skeleton. In the fermentation, macrocyclic β-caryophyllene was remodeled into a series of natural product-like polycyclic compounds with unusual diverse skeletons (1–7). Their structures, including absolute configuration, were elucidated by several spectroscopic methods (1D & 2D NMR, HRMS, ECD and X-ray diffraction). Especially, scaffolds 1 and 3 were not found in acid-catalysed products before. Our findings demonstrated the potential of endophytic fungi for catalyzing macrocyclic compounds into diverse products.
Introduction
Due to their complexity and diversity, natural products (NP) have become prime resources for drug leads.1 But the current collection of NP is limited by elaborate isolation procedures or lengthy total syntheses. Recently, diversity-oriented synthesis (DOS) has emerged as an efficient methodology to access chemically diverse libraries.2–4 Especially, complex NP were selected as starting points to generate NP-like scaffolds.5,6 Futhermore, transannular or Wagner–Meerwein rearrangements on terpenoids yield a wide variety of natural skeletons. Consequently, in our opinion, cascade transannular rearrangement on macrocyclic NP will be a very useful approach leading to highly diverse skeletons.The macrocyclic sesquiterpene, (−)-β-caryophyllene, is a major sesquiterpene component in the essential oils of plants (esp. in cloves)7–9 and microorganisms.10–15 Due to its strained cyclobutane, macrocyclic moiety and exocyclic olefin, β-caryophyllene acts as a key precursor in nature to form diverse tricyclic sesquiterpenes by transannular rearrangements.16
In our continuing work on NP-based biotransformation,17–20 β-caryophyllene was selected as starting point to generate a NP-like library with diverse skeletons. Especially, plant endophytes were considered as suitable partners for the biosynthesis of NPs. To further expand the structural diversity from caryophyllene and investigate the potential of employing endophytes as biocatalysts, an endophytic strain, Aspergillus tubingensis KJ-9, was screened in our lab to catalyse transannular rearrangement of the β-caryophyllene skeleton. HPLC profiles (Fig. S1†) indicated that the strain KJ-9 yields unusual metabolites when it was fermented with β-caryophyllene. The fermentations were then separated by repeated column chromatography to yield seven previously unreported sesquiterpenes (1–7) with five different backbones, including an unprecedented skeleton 1 (Scheme 1).
Results and discussion
Compound 1 was assigned molecular formula (MF) C15H22O2 by HR ESIMS (m/z 257.1508 [M + Na]+), with five degrees of unsaturation. Its 13C and DEPT NMR spectroscopic data (Fig. S2†) revealed the presence of an unsaturated ketone (δC 162.2, 127.0 and δC 199.8). Apart from those two degrees of unsaturated bonds, the remaining implied that 1 possesses three rings in the structure. Extensive analysis of the 2D NMR correlations (HSQC, 1H–1H COSY, and HMBC, as shown in Fig. 1a) established the whole planar structure. 1H–1H COSY data of 1 showed one isolated spin system of H2-2/H-1/H2-9. HMBC correlations from H3-15 to C-1, C-10, and C-14 indicated C-15, C-14, C-1 and C-10 linked up through the quaternary C-11. Correlations from H3-12 to C-3, C-4 and C-5 confirmed the methyl C-12, C-3 and C-5 linked up at the olefinic C-4. The HMBC correlations of H2-10/C-8, H2-9/C-3, H3-13/C-8 and H3-13/C-9 revealed a bicyclo[2.2.1]heptane moiety. Furthermore, the correlations of H3-13 to C-7 and C-8, H2-7 to C-5 and C-6 indicated a cyclohex-2-en-1-one substructure. Thus, the whole planar structure of 1 was elucidated as a novel 5/5/6 sesquiterpene skeleton.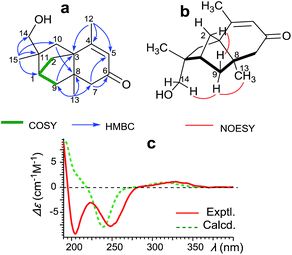 | ||
| Fig. 1 Structure elucidation of 1. (a) Key correlations in COSY & HMBC; (b) key correlations in NOESY; (c) experimental and theoretical CD spectra of (1S,3S,8R,11R)-1 (in MeCN). | ||
The relative configuration (RC) of 1 was deduced from NOESY correlations (Fig. 1b). The NOE signals among H-14b, H-9α and H3-13 indicated that C-14 and Me-13 are on the same side, which were arbitrarily assigned as α-orientation. An NOE correlation of H-9β/H-2 indicated that the bridge C-2 is in the β-orientation. Since all the products were derived from bioconversion of caryophyllene through complex arrangements, it is necessary to determine their absolute configuration (AC) on the basis of unambiguous experimental proof. The experimental CD spectrum was compared with the theoretical curve calculated by TDDFT method.21–23 The calculated CD curve for (1S,3S,8R,11R)-1 (Fig. 1c) showed the same positive Cotton effect as the experimental around 290 nm. Thus, its AC was established therein.
MF of 2, C15H22O2, was deduced from HR ESIMS (m/z 257.1504 [M + Na]+), and has five degrees of unsaturation. Its 13C NMR data indicate a double bond and a carbonyl in its structure. The planar structure was elucidated on the basis of COSY and HMBC correlations, as shown in Fig. 2a, possessing a similar skeleton as that of α-neoclovene, a rearranged product from caryophyllene.24,25 However, this is the first report of NMR data for this type of skeleton. Its RC was deduced by NOESY correlations as shown in Fig. 2b. The NOE correlations of H-15/H-1 hydroxymethyl C-14 and the bridge carbons C-1 and C-2 are on the same β side. The NOE cross peaks of H3-14/H-10α/H3-13 indicated that both methyls C-13 and C-14 are on the same α side. The theoretical CD curve of (3R,8S,9R,11S)-2 stereoisomer yield a similar curve as that of experimental as shown in Fig. 2c. Both calculated and experimental CD spectra showed negative Cotton effect around 225 nm and positive transition around 290 nm. Furthermore, the assigned AC is also consistent with the previously reported analogs.24,25
 | ||
| Fig. 2 Structure elucidation of 2. (a) COSY & HMBC; (b) NOESY; (c) experimental and theoretical CD spectra of (3R,8S,9R,11S)-2 (in MeCN). | ||
Compound 3 had the same MF as that of 1 or 2, as deduced by HR ESIMS (m/z 257.1505 [M + Na]+). The 1H and 13C NMR data suggest an unsaturated ketone moiety in the molecule. Detailed analysis of the 2D NMR (Fig. 3a) revealed its core skeleton. The structure was similar to that of a product from H+/MeCN,26 except for the cyclohex-2-en-1-one substructure. Its RC was deduced from NOE correlations in the NOESY spectrum (Fig. 3b). The Me-15 was arbitrarily assigned as β-orientation. The NOE correlations of H3-15/H-2 indicated the carbonic bridge C-2/C-3 on the β side. The NOE correlation of H3-13/H-10α indicted the methyl C-13 on the α side. Since the bridge C-2/C-3 connect C-1/C-4, respectively, the C-4 is forced in the β-orientation. Therefore, the whole RC was assigned as the model in Fig. 3b. Its AC was determined by comparing its experimental CD curve with the theoretical one. The calculated CD spectrum of (1S,4R,8S,9S)-3 by TDDFT method has the same positive Cotton effect around 220 nm to that of experimental CD curve (Fig. 3c). Thus, the AC of 3 was determined as 1S,4R,8S,9S.
 | ||
| Fig. 3 Structure elucidation of 3. (a) COSY & HMBC; (b) NOESY; (c) experimental and theoretical CD spectra of (1S,4R,8S,9S)-3 (in MeCN). | ||
1D NMR data (Fig. S2†) and 2D NMR correlations of compound 4 (Fig. 4a) revealed a 4/5/6 skeleton, the same as the core skeletons of naematolins C and G from the fungus Naematoloma fasciculare.27 Its RC was deduced by NOESY correlations (Fig. 4b). Compound 5 possesses similar 1H and 13C NMR data and 2D NMR correlations to those of 4 with only one exceptional methoxyl ester group (δC 53.1, 178.6). The group was assigned at C-15 based on the HMBC correlations of MeO/C-15 and H3-14/C-15. Similar NOESY correlations and the same Cotton effect around 215 nm (Fig. 4c) between 4 and 5 confirmed that they also possess the same AC. The calculated CD spectrum of (1R,3S,8S,9R,11R)-5 stereoisomer displayed the same negative Cotton effects with that of the experimental one (Fig. 4c). Thus, their ACs were assigned therein.
 | ||
| Fig. 4 Structure elucidation of 4. (a) COSY & HMBC; (b) NOESY; (c) experimental and theoretical CD spectra of (1R,3S,8S,9R,11R)-5 (in MeCN). | ||
The 2D NMR correlations (HSQC, COSY and HMBC) as shown in Fig. 5a of compound 6 revealed a 4/7/6 skeleton. Its planar structure and RC were further confirmed by X-ray diffraction (Fig. 5b, CCDC no. 1413642). Since the compound does not contain any chromophore, its CD cannot give rise to reliable Cotton effect to assign its AC. However, the stereochemistry of two chiral centers C-1 and C-9 kept unchanging after the arrangement. As a result, the configurations of C-4 and C-8 can be deduced from X-ray analysis. The X-ray model indicated that the C-12 bridge and C-15 are on the β side, the same as H-1. Therefore, the AC of 6 was assigned as 1R,4R,8S,9S,11R.
Compound 7 shows very similar chemical shifts in its 1H and 13C NMR spectra compared to those of 6, apart from a methoxyl substitution in 7. Its MF of C16H26O2, deduced from HR EIMS, also indicated 7 has one more MeO group. HMBC correlations of 7 from the MeO to C-4 indicated the position of the MeO group attached at C-4.
In the formation of those rearranged scaffolds, the double bond at C-8/C-13 shifted to C-8/C-9. The transannular cyclizations of β-caryophyllene initiated by electrophilic attack on the shifted double bond (Scheme 2). In the formation of 1, the cyclobutane moiety opens to yield a carbonium ion at C-10 (i), which then attacks to the double bond at C-4/C-5 to form the skeleton of 1. Then, elimination and oxidation reactions on the carbonium ion ii gave the product 1. The formation of 3 also achieved, via transannular rearrangement, elimination and oxidation reactions. The other scaffolds (2 and 4–7) are typical skeletons derived from caryophyllene. Their formations can be referred to a previous review.16
Conclusions
Our findings demonstrated the potential of endophytic fungi catalysing macrocyclic sesquiternoid β-caryophyllene to afford NP-like polycyclic compounds containing novel skeletons. Among them, skeleton 4 was also found in nature. Notably, scaffolds of 1 and 3 have not yet been acquired by acid catalysis. Compared to catalysis by acid or Lewis acid, the fungus KJ-9 promoted even more chemical diversity by oxidizing gem-dimethyls and allylic positions on the arranged skeletons. In addition, β-caryophyllene is abundant in natural sources, so it can serve as a synthon to construct complex cyclic scaffolds covering unknown chemical space or bioactive space. On the basis of the current findings, combination of caryophyllene with other naturally bioactive blockers will produce more diverse products with NP-like scaffolds.In fact, nature always knows best. For example, a medicinal plant Psidium guajava (guava) selects β-caryophyllene and another bioactive block to generate an α-glucosidase inhibitor, a novel caryophyllene-based meroterpenoid, guajadial, with higher activity than that of the drug acarbose.28–31 In our lab, a biomimic synthesis is being carried out to combine β-caryophyllene and another NP pharmacophore, which will generate a novel chemical library by rearrangement in order to cover unknown chemical space with high bioactivities.
Acknowledgements
This work is financially supported from the National Natural Science Foundation of China (No. 21102114).Notes and references
- J. W.-H. Li and J. C. Vederas, Science, 2009, 325, 161 CrossRef PubMed.
- V. A. Ignatenko, Y. Han and G. P. Tochtrop, J. Org. Chem., 2013, 78, 410 CrossRef CAS PubMed.
- B. R. Balthaser, M. C. Maloney, A. B. Beeler, J. A. Porco and J. K. Snyder, Nat. Chem., 2011, 3, 969 CrossRef CAS PubMed.
- N. Kumar, M. Kiuchi, J. A. Tallarico and S. L. Schreiber, Org. Lett., 2005, 7, 2535 CrossRef CAS PubMed.
- H. Kikuchi, K. Sakurai and Y. Oshima, Org. Lett., 2014, 16, 1916 CrossRef CAS PubMed.
- G. Appendino, G. C. Tron, T. Jarevång and O. Sterner, Org. Lett., 2001, 3, 1609 CrossRef CAS PubMed.
- J. T. Knudsen, L. Tollsten and L. G. Bergström, Phytochemistry, 1993, 33, 253 CrossRef CAS.
- I. Kubo, S. K. Chaudhuri, Y. Kubo, Y. Sanchez, T. Ogura, T. Saito, H. Ishikawa and H. Haraguchi, Planta Med., 1996, 62, 427 CrossRef CAS PubMed.
- J. R. Hanson, Nat. Prod. Rep., 2001, 18, 607 RSC.
- M. Wichlacz, W. A. Ayer, L. S. Trifonov, P. Chakravarty and D. Khasa, J. Nat. Prod., 1999, 62, 484 CrossRef CAS PubMed.
- S.-W. Yang, T.-M. Chan, J. Terracciano, E. Boehm, R. Patel, G. Chen, D. Loebenberg, M. Patel, V. Gullo, B. Pramanik and M. Chu, J. Nat. Prod., 2009, 72, 484 CrossRef CAS PubMed.
- W.-J. Wang, D.-Y. Li, Y.-C. Li, H.-M. Hua, E.-L. Ma and Z.-L. Li, J. Nat. Prod., 2014, 77, 1367 CrossRef CAS PubMed.
- M. Pulici, F. Sugawara, H. Koshino, J. Uzawa, S. Yoshida, E. Lobkovsky and J. Clardy, J. Org. Chem., 1996, 61, 2122 CrossRef CAS.
- Y. Li, S. Niu, B. Sun, S. Liu, X. Liu and Y. Che, Org. Lett., 2010, 12, 3144 CrossRef CAS PubMed.
- Q.-Y. Qi, L. Bao, J.-W. Ren, J.-J. Han, Z.-Y. Zhang, Y. Li, Y.-J. Yao, R. Cao and H.-W. Liu, Org. Lett., 2014, 16, 5092 CrossRef CAS PubMed.
- I. G. Collado, J. R. Hanson and A. J. Macías-Sánchez, Nat. Prod. Rep., 1998, 15, 187 RSC.
- G.-W. Wu, J.-M. Gao, X.-W. Shi, Q. Zhang, S.-P. Wei and K. Ding, J. Nat. Prod., 2011, 74, 2095 CrossRef CAS PubMed.
- G. W. Wu, X. J. Li, Y. Gao, Y. Jia and J. M. Gao, Chin. Chem. Lett., 2010, 21, 446 CrossRef CAS PubMed.
- T. Dong, G.-W. Wu, X.-N. Wang, J.-M. Gao, J.-G. Chen and S.-S. Lee, J. Mol. Catal. B: Enzym., 2010, 67, 251 CrossRef CAS PubMed.
- C. Yang, H. Fan, Y. Yuan and J. Gao, Chin. J. Chem., 2013, 31, 127 CrossRef CAS PubMed.
- M. Xue, Q. Zhang, J.-M. Gao, H. Li, J.-M. Tian and G. Pescitelli, Chirality, 2012, 24, 668 CrossRef CAS PubMed.
- J.-M. Gao, J.-C. Qin, G. Pescitelli, S. Di Pietro, Y.-T. Ma and A.-L. Zhang, Org. Biomol. Chem., 2010, 8, 3543 CAS.
- S.-X. Yang, J.-M. Gao, H. Laatsch, J.-M. Tian and G. Pescitelli, Chirality, 2012, 24, 621 CrossRef CAS PubMed.
- W. Parker, R. A. Raphael and J. S. Roberts, Tetrahedron Lett., 1965, 6, 2313 CrossRef.
- H. Iwabuchi, M. Yoshikura, Y. Ikawa and W. Kamisako, Chem. Pharm. Bull., 1987, 35, 1975 CrossRef CAS.
- O. I. Yarovaya, D. V. Korchagina, T. V. Rybalova, Y. V. Gatilov, M. P. Polovinka and V. A. Barkhash, Russ. J. Org. Chem., 2004, 40, 1593 CrossRef CAS.
- K. Doi, T. Shibata, N. Yokoyama, H. Terasawa, O. Matsuda and S. Kashino, J. Chem. Soc., Chem. Commun., 1990, 725 RSC.
- X.-L. Yang, K.-L. Hsieh and J.-K. Liu, Org. Lett., 2007, 9, 5135 CrossRef CAS PubMed.
- M. Shao, Y. Wang, Z. Liu, D.-M. Zhang, H.-H. Cao, R.-W. Jiang, C.-L. Fan, X.-Q. Zhang, H.-R. Chen, X.-S. Yao and W.-C. Ye, Org. Lett., 2010, 12, 5040 CrossRef CAS PubMed.
- Y. Ou, X. Zhu, R. Liu, X. Liu, L. Su, L. Xie and Y. Cao, J. Hunan Univ., 2014, 34, 17 CAS.
- H.-Z. Fu, Y.-M. Luo, C.-J. Li, J.-Z. Yang and D.-M. Zhang, Org. Lett., 2010, 12, 656 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and calculation procedures, spectra data, NMR, IR and HRMS spectra of compounds 1–7. See DOI: 10.1039/c5ra14243a |
| This journal is © The Royal Society of Chemistry 2015 |



