Enhancement of sinterability and mechanical properties of B4C ceramics using Ti3AlC2 as a sintering aid
Y. Q. Tana,
C. Chena,
F. Z. Lia,
H. B. Zhang*a,
G. J. Zhang*b and
S. M. Peng*a
aInnovation Research Team for Advanced Ceramics, Institute of Nuclear Physics and Chemistry, China Academy of Engineering Physics, Mianyang 621900, China. E-mail: hbzhang@caep.cn; gjzhang@mail.sic.ac.cn; pengshuming@caep.cn
bShanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China
First published on 3rd September 2015
Abstract
Boron carbide (B4C) ceramics are currently the leading control rod materials in fast nuclear reactors and promising high temperature structural materials. However, several drawbacks such as poor sinterability and low toughness seriously limit their wide applications. In order to enhance the sinterability and mechanical properties of B4C ceramics, titanium aluminum carbide (Ti3AlC2) was chosen as a new efficient sintering aid to densify B4C ceramics using spark plasma sintering. Fully dense B4C ceramics were obtained at a lower sintering temperature of 1500 °C by adding a small amount of Ti3AlC2. Meanwhile, the mechanical properties were enhanced remarkably. For B4C ceramics sintered with 15 vol% Ti3AlC2, optimized mechanical properties were obtained with Vickers hardness of 40.2 GPa and indentation fracture toughness of 4.7 MPa m1/2. These results indicate that Ti3AlC2 can be used as a novel sintering aid for the densification of B4C ceramics.
Introduction
Boron carbide (B4C) ceramics are excellent structural materials due to their low density (2.52 g cm−3), high Young's modulus (∼440 GPa) and high Vickers hardness (∼30 GPa),1,2 which make B4C ceramics excellent candidates for light-weight body armors. B4C ceramics also have great potential for high temperature applications in non-oxidizing environments owing to their high melting point and good chemical stability. Besides, B4C ceramics are important functional materials due to the neutron absorption ability of isotope 10B which owns a high neutron absorption cross-section over a wide range of neutron energies. Because of their high B content, good chemical inertness and good mechanical properties, B4C ceramics are extensively used in the nuclear reactors as control rods, shielding materials and as neutron detectors. Currently, they are the leading control rod materials in liquid-metal-cooled fast breeder reactors (LMFBR) and boiling water reactors (BWR).3However, there exist several major restrictions for the wide applications of B4C ceramics as both structural and functional materials: (a) poor sinterability due to the highly covalent bonding and the associated low diffusion mobility; (b) low machinability due to high hardness and low electrical conductivity; (c) relatively low strength and toughness.1 Further, as control rod materials in nuclear reactors, B4C ceramics often experience serious cracking when subjected to thermal stresses and helium gas due to their intrinsic low thermal conductivity, low toughness and poor thermal shock resistance.4
The poor sinterability makes the preparation and processing of B4C ceramics rather complicated and energy-consuming. The relatively low toughness and low thermal conductivity of B4C ceramics could influence the lifetime of control rods and raise safety problems to the nuclear reactors.5 Thus it is essential to lower the sintering temperature and enhance the mechanical properties of B4C ceramics for wider applications and the extension of lifetime of control rods in nuclear reactors. Many efforts have been paid to lower the sintering temperature of B4C ceramics and improve their mechanical properties. For the reduction of sintering temperature, carbon (C),6–8 metallic phase such as aluminium (Al),9–11 silicon (Si),12,13 and titanium (Ti),14 oxides such as alumina (Al2O3) and rare earth oxides,15,16 as well as borides17 were commonly chosen as sintering aids. C is effective in reducing the sintering temperature of pressureless sintered B4C because C can remove the oxide layer of B4C particles and enhance the diffusion at the grain boundaries.6–8 The other sintering aids mentioned above generally form liquid phase due to their lower melting points and thus promote densification of B4C ceramics significantly.9–17 Although these sintering aids can facilitate sintering of B4C ceramics at a much lower temperature, in most cases, no remarkable improvements on mechanical properties of B4C ceramics were achieved. Generally, the residual carbon or metallic phases at the grain boundaries generally deteriorate the unique properties of hard ceramics. Moreover, abnormal grain growth caused by liquid phase during sintering could lead to lower toughness of ceramics. In order to increase the strength and toughness of B4C ceramics, several additives have been explored such as TiB2, ZrB2 and SiC,11,18–22 among which TiB2 is the most extensively studied. The B4C–TiB2 composites can be obtained by direct mixing of B4C and TiB2,11 or by in situ reaction sintering between B4C and TiC or TiO2 and C.18–20 Besides, carbon nanotube (CNT)9 and carbon nanofiber (CNF)23 reinforced B4C ceramics were also reported. The introduction of TiB2, CNT or CNF could result in a remarkable increase of the mechanical properties of B4C ceramics. Generally, B4C–TiB2 composites show an improved fracture toughness in the range of 3–6 MPa m1/2 depending on the amount of TiB2 and different sintering methods.11,18–20 B4C–CNF composite was reported to exhibit an extremely high fracture toughness of 7.5 MPa m1/2 when the volume fraction of CNF was 15%.23 However, the enhancement of sinterability for B4C ceramics is quite limited. The sintering temperature of B4C–TiB2 composites still needs to be around 2000 °C and above by reaction hot-pressing of B4C, TiO2 and C powders.19
In order to enhance the sinterability and mechanical properties of B4C ceramics simultaneously, titanium aluminum carbide (Ti3AlC2), a typical member of the MAX phases, was chosen as a sintering aid to prepare dense B4C ceramics in this study. The densification was carried out by spark plasma sintering which is an effective way to fabricate dense nanostructured ceramics. The influence of Ti3AlC2 content on the sinterability and mechanical properties of B4C ceramics was systematically studied.
Experimental
B4C powders (Grade HS, H. C. Starck GmbH, Germany) and Ti3AlC2 powders (>99%, Forsman, China) were used as starting materials. Fig. 1 shows the microstructures of these two raw materials. The B4C powder has a uniform grain size distribution with an average particle size of 0.5 μm and the Ti3AlC2 powder used in this study shows a broad grain size distribution from 0.5 μm to about 10 μm, as shown in Fig. 1. B4C and Ti3AlC2 powders were mixed according to the formula of B4C-x vol% Ti3AlC2 with x = 0, 5, 10, 15 and 20. The mixed powders were ball-milled for 10 h with SiO2 balls using ethanol as a ball-milling media. After ball-milling, the mixed powders were dried and then put into a carbon die with an inner diameter of 15 mm. B4C-x vol% Ti3AlC2 mixtures were sintered in vacuum using an SPS furnace (Labox-325, Japan) for 3 minutes at different temperatures. The heating rate is 100 °C min−1 and a uniaxial pressure of 80 MPa was applied during sintering.The density of the sintered samples was measured by the Archimedes's method. Crystalline phases were characterized by X-ray diffraction (XRD). The microstructure of the specimens was observed using scanning electron microscopy (SEM) with an energy-dispersive X-ray (EDX) analyser. In order to measure the Vickers hardness (HV) and fracture toughness (KIC), specimens were first polished with diamond slurry. Hardness was determined by the Vickers diamond indentation method using the following equation:
| Hv = 1.854 × 109P/d2 | (1) |
| KIC = 0.016(E/Hv)1/2(P/c3/2) | (2) |
Results and discussion
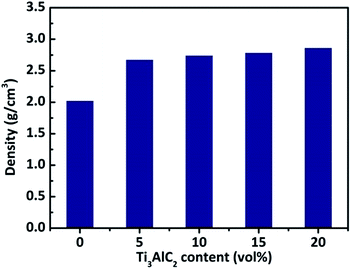
Fig. 2 shows the density of B4C ceramics sintered with different amount of Ti3AlC2 at 1500 °C. The density of monolithic B4C ceramics spark plasma sintered at 1500 °C is only 2.02 g cm−3, which is about 80.1% of the theoretical density. When 5 vol% Ti3AlC2 was added, the density of B4C increased dramatically to 2.69 g cm−3. Further increase in Ti3AlC2 content did not bring to any appreciable density increase. 5 vol% of Ti3AlC2 was therefore enough to obtain a fully dense B4C ceramic at 1500 °C. When the sintering temperature was increased to 1600 °C, the relative density of monolithic B4C ceramics increased to 98.0%. This indicates that the lowest sintering temperature for fully dense B4C ceramics is at least 100 °C lower when spark plasma sintered using Ti3AlC2 as a sintering aid, which suggests that Ti3AlC2 is quite effective in lowering the sintering temperature of B4C ceramics.The phase composition of B4C ceramics sintered with different amount of Ti3AlC2 was examined by XRD analysis and the results are shown in Fig. 3. For monolithic B4C ceramics, only diffraction peaks of single phase B4C were detected. B4C ceramics sintered with Ti3AlC2 show several additional diffraction peaks besides those corresponding to B4C. However, no Ti3AlC2 diffraction peaks were observed, demonstrating that Ti3AlC2 decomposed completely at high temperature. The existence of TiB2 can be identified by the diffraction peaks labelled in Fig. 4, and it can be seen that the intensity of the main TiB2 diffraction peak around 45° increases gradually with the increase of Ti3AlC2 content. A broad peak around 26° indicates the existence of C and its intensity also increases with the increase of Ti3AlC2 amount. Further, a small peak around 33° which belongs to Al2OC phase was observed for all B4C ceramics sintered with Ti3AlC2. For B4C ceramics sintered with 20 vol% Ti3AlC2, one more set of additional diffraction peaks was detected. An accurate peak matching revealed that it corresponds to the Al–B–C phase Al8B4C7, which was also observed in previous B4C ceramics sintered using Al as sintering aid.7,13 Based on the XRD results and previous studies, it can be summarized that the Ti3AlC2 first decomposed according to the following reaction:25
| Ti3AlC2 → 3TiC2/3 + Al | (3) |
Then TiC2/3 and Al reacted with B4C respectively during sintering according to the following reactions:26
| 3B4C + 6TiC2/3 → 6TiB2 + 7C | (4) |
| 8Al + B4C + 6C → Al8B4C7 | (5) |
As to the Al2OC phase, it is suggested that the oxygen may come from the oxide layer of B4C particles. Generally, the outer surface of B4C fine particle is covered by an oxide layer of B2O3, which is one reason for the low sinterability of B4C ceramics.1,7 In this study, it is suggested that Al decomposed from Ti3AlC2 and the residual C from the above reactions can remove this oxidized layer and form Al2OC phase according to the following reactions:
| 2Al + B2O3 → Al2O3 + 2B | (6) |
| Al2O3 + 3C → Al2OC + 2CO | (7) |
Fig. 4 shows the microstructures observed in the polished surface of B4C ceramics sintered with different amount of Ti3AlC2 and the typical EDS spectra of B4C ceramics sintered with 15 vol% Ti3AlC2. Fig. 4(a) and (b) are polished surfaces of monolithic B4C ceramics sintered at 1500 °C and 1600 °C respectively; Fig. 4(c)–(f) correspond to B4C ceramics sintered at 1500 °C with 5, 10, 15 and 20 vol% Ti3AlC2 respectively. Monolithic B4C ceramic sintered at 1500 °C shows a porous microstructure, which is consistent with its low density. When sintered at 1600 °C, B4C ceramic becomes much denser as shown in Fig. 4(b). All the B4C ceramics sintered at 1500 °C with Ti3AlC2 as sintering aid show quite dense microstructures. With the addition of Ti3AlC2, a white contrasted phase uniformly dispersed in the B4C matrix starts to appear, indicating the presence of second phases. The size of these white spots varies from 0.5 μm to about 10 μm, which is consistent with the size distribution of raw Ti3AlC2 powders. The volume fraction of these white spots was estimated from the polished surfaces using the ImageJ software and it is calculated to be 6.2%, 13.8%, 17.4% and 24.9% for B4C ceramics sintered with 5, 10, 15 and 20 vol% Ti3AlC2 respectively. These values are roughly consistent with the volume fractions of the secondary phases proposed by eqn (3)–(5) for B4C ceramics sintered with different amount of Ti3AlC2.
In order to present a better understanding of the distribution of different phases in the B4C matrix, quantitative analysis of the second phases was done by SEM with EDS analyser on the polished surface of B4C ceramics sintered with different amount of Ti3AlC2. As shown in Fig. 4, the grey area is the main B4C phase, and the white spots indicated with white arrows are TiB2 according to the EDS analysis (Fig. 4(h)). Besides the white spots, there also exist some white areas which have no clear boundaries as indicated in the blue circles of Fig. 4(c)–(f). The EDS spectrum demonstrates that they are Al–B–C phases (Fig. 4(h)), and referring to the XRD results they should be Al8B4C7. It was supposed that Al originally existed at the brighter contrasted areas, and then the molten Al diffused into surroundings during sintering. The light grey spots indicated with red arrows in Fig. 4(e) and (f) and area D in Fig. 4(g) are Al–O–C phase, presumably Al2OC (Fig. 4(h)).
From the above experimental results, it can be summarized that the mechanisms for the enhancement of sinterability of B4C ceramics using Ti3AlC2 as a sintering aid are many-sided. Firstly, Ti3AlC2 can decompose into Al and TiC2/3 at high temperature, which has been confirmed by previous study.25 The Al decomposed from Ti3AlC2 can form liquid phase at high temperature and promote sintering effectively. Secondly, Al can remove the oxide layer of B4C particles. The elimination of oxides layer on the surface of initial B4C powders allows direct contact between B4C particles, permitting sintering to initiate at significantly lower temperature,1 thereby accelerating the densification of B4C. Thirdly, the in situ reaction sintering between B4C and TiC2/3 facilitates mass transfer in the aggregate and the enhancement of mass transport could allow the densification to occur at lower temperatures where the grain growth is still limited. The residual C produced by the reaction between B4C and TiC2/3 could further removes the oxide layer. Besides, it has been confirmed that the presence of TiB2 could result in lowering of activation energy for sintering of B4C through various machnisms.28 The increased sinterability has been reported in the B4C–TiB2 composites through reaction hot-pressing of B4C and TiC.29 Therefore, the improved sinterability of B4C ceramics using Ti3AlC2 as sintering aid is a consequence of liquid phase sintering, removal of oxide layer and in situ reactive sintering. The reactive sintering and the presence of TiB2 and C could also inhibit grain growth effectively, thereby giving rise to fine and uniform grain size distributions.
Fig. 5 shows the typical optical micrographs of the Vickers hardness indents and the induced cracks of B4C ceramics sintered with different amounts of Ti3AlC2. Fig. 5(a) and (b) correspond to monolithic B4C sintered at 1500 °C and 1600 °C respectively, and Fig. 5(b)–(d) correspond to B4C ceramics sintered at 1500 °C with 5, 10, 15 and 20 vol% Ti3AlC2 respectively. It can be seen that all ceramics show clear indentations. Monolithic B4C ceramics exhibit long and straight cracks induced by the indentation. In case of B4C ceramics sintered with Ti3AlC2, the cracks become shorter and more curved and the indentation becomes smaller.
The Vickers hardness and indentation fracture toughness of all these B4C ceramics were calculated according to the length of the indentations and the induced cracks, and the results are shown in Fig. 6. The filled symbols represent B4C ceramics sintered at 1500 °C with different amount of Ti3AlC2, while the open symbols represent monolithic B4C ceramics sintered at 1600 °C. Monolithic B4C ceramic sintered at 1500 °C shows a low Vickers hardness of 12 GPa due to its high porosity. The Vickers hardness of monolithic B4C ceramic increases dramatically to 37.6 GPa when sintered at 1600 °C. In case of B4C ceramics sintered with Ti3AlC2, only a slight increase of Vickers hardness is obtained compared to fully dense monolithic B4C ceramics. With increasing the amount the Ti3AlC2, the Vickers harness of B4C ceramics keeps almost the same around 40 GPa, which is higher than most of the B4C ceramics and B4C–TiB2 composites reported1,11 and close to the values of superhard B4C–ZrB2 ceramics.30,31 Porosity and grain size are two most important factors that can influence the Vickers hardness of B4C ceramics. In this study, except the monolithic ceramic sintered at 1500 °C, all the other B4C ceramics have high density with only negligible porosity. Besides, SPS could suppress the grain growth effectively, especially when using Ti3AlC2 as a sintering aid. Thus the low porosity and fine grain size (around 0.5 μm) give rise to high Vickers hardness in this study. Different from the Vickers hardness, the fracture toughness of B4C ceramics changes dramatically with the increase of Ti3AlC2 content. For dense monolithic B4C ceramics, the indentation fracture toughness is only 2.32 MPa m1/2, which is in good consistency with previous reports.1,11 It increases gradually with increasing Ti3AlC2 content and a maximum value of 4.7 MPa m1/2 is achieved for B4C ceramics sintered with 10 and 15 vol% Ti3AlC2. The increase of indentation fracture toughness can be ascribed to following two main factors: a well sintered fine-grained B4C matrix and uniformly distributed TiB2 particles. The different thermal expansion coefficients of TiB2 (α = 8.7 × 10−6 K−1) and B4C (α = 5.7 × 10−6 K−1) generate residual stress around TiB2 particles during cooling and this residual stress gives rise to the microcracks and thus the crack deflection.20 In this study, the propagation of cracks was hindered by the dispersion of TiB2 particles and the higher fracture toughness in B4C ceramic sintered with 10 and 15 vol% Ti3AlC2 can be explained by the crack deflection by TiB2 particles, as demonstrated in Fig. 7. With further increase of Ti3AlC2 content to 20 vol%, the fracture toughness of B4C ceramics decreases slightly. The increase of Al-rich phases is one possible reason. For B4C ceramics sintered with 20 vol% Ti3AlC2, some aggregation of TiB2 particles are shown in Fig. 4(f), and the aggregation of TiB2 phases could be responsible for the decrease of fracture toughness.
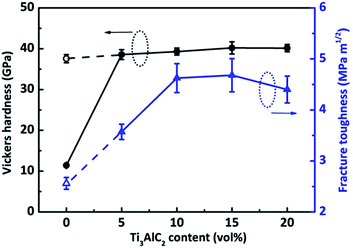 | ||
| Fig. 6 Variations of Vickers hardness and fracture toughness of B4C ceramics with the amount of Ti3AlC2 additive, hollow markers refer to monolithic B4C sintered at 1600 °C. | ||
 | ||
| Fig. 7 Demonstration of crack deflection in B4C ceramics sintered with (a) 15 vol% and (b) 20 vol% Ti3AlC2, the arrows indicate the crack propagation directions. | ||
Conclusions
Fully dense B4C ceramics were obtained at a lower sintering temperature using Ti3AlC2 as a novel sintering aid in this study, meanwhile their mechanical properties were enhanced. The B4C ceramics sintered with 15 vol% Ti3AlC2 exhibits the maximized mechanical properties with Hv = 40.2 GPa and KIC = 4.7 MPa m1/2. The mechanisms for the enhancement of sinterability include liquid phase of aluminium decomposed from Ti3AlC2 at high temperatures, removal of surface oxide layer B2O3 by C and Al and reaction sintering between B4C and TiC2/3. The enhancement of mechanical properties was mainly attributed to grain refinement and crack deflections owing to the presence of TiB2 particles.Acknowledgements
Haibin Zhang is grateful to the foundation by the Recruitment Program of Global Youth Experts and the Youth Hundred Talents Project of Sichuan Province. This work is supported by the National Natural Science Foundation of China (Grant no. 91326102), the Science and Technology Development Foundation of China Academy of Engineering Physics (Grant no. 2013A0301012), and Science and Technology Innovation Research Foundation of Institute of Nuclear Physics and Chemistry.References
- A. K. Suri, C. Subramanian, J. K. Sonber and T. S. R. C. Murthy, Int. Mater. Rev., 2010, 55, 1 CrossRef PubMed.
- V. Domnich, S. Reynaud, R. A. Haber and M. Chhowalla, J. Am. Ceram. Soc., 2011, 94, 3605 CrossRef CAS PubMed.
- F. Thevenot, J. Eur. Ceram. Soc., 1990, 6, 205 CrossRef CAS.
- T. Stoto, N. Housseau, L. Zuppiroli and B. Kryger, J. Appl. Phys., 1990, 68, 3198 CrossRef CAS PubMed.
- T. Maruyama, J. Tech. Assoc. Refract., Jpn., 2010, 30, 80 CAS.
- K. A. Schwetz and W. Grellner, J. Less-Common Met., 1981, 82, 37 CrossRef CAS.
- B. Y. Yin, L. S. Wang and Y. C. Fang, J. Chin. Ceram. Soc., 2001, 29, 68 CAS.
- H. Lee and R. F. Speyer, J. Am. Ceram. Soc., 2003, 86, 1468 CrossRef CAS PubMed.
- T. Kobayashi, K. Yoshida and T. Yano, J. Nucl. Mater., 2013, 440, 524 CrossRef CAS PubMed.
- C. H. Danny, J. P. Aleksander, A. A. Ilhan and E. S. William, J. Am. Ceram. Soc., 1989, 72, 775 CrossRef PubMed.
- M. Mashhadi, E. Taheri-Nassaj, M. Mashhadi and V. M. Sglavo, Ceram. Interfaces, 2011, 37, 3229 CrossRef CAS PubMed.
- F. Ye, Z. Hou, H. Zhang and L. Liu, J. Am. Ceram. Soc., 2010, 93, 2956 CrossRef CAS PubMed.
- C. Xu, H. Zeng and G. Zhang, Int. J. Refract. Met. Hard Mater., 2013, 41, 2 CrossRef CAS PubMed.
- R. Telle and G. Petzow, Powder Metall., 1986, 2, 1155 CAS.
- C. H. Lee and C. H. Kim, J. Mater. Sci., 1992, 27, 6335 CrossRef CAS.
- K. Y. Xie, M. F. Toksoy, K. Kuwelkar, B. Zhang, J. A. Krogstad, R. A. Haber and K. J. Hemker, J. Am. Ceram. Soc., 2014, 97, 3710 CrossRef CAS PubMed.
- S. Yamada, K. Hirao, Y. Yamachi and S. Knzaki, J. Mater. Sci. Lett., 2002, 21, 1445 CrossRef CAS.
- A. Goldstein, Y. Yeshurun and A. Goldenberg, J. Eur. Ceram. Soc., 2007, 27, 695 CrossRef CAS PubMed.
- S. Yamadaa, K. Hiraob, Y. Yamauchib and S. Kanzakib, J. Eur. Ceram. Soc., 2003, 23, 1123 CrossRef.
- V. Skorokhod and V. D. Krstic, J. Mater. Sci. Lett., 2000, 19, 237 CrossRef CAS.
- A. Goldstein, Y. Geffen and A. Goldenberg, J. Am. Ceram. Soc., 2001, 84, 642 CrossRef CAS PubMed.
- Z. Zhang, X. Du, Z. Li, W. Wang, J. Zhang and Z. Fu, J. Eur. Ceram. Soc., 2014, 34, 2153 CrossRef CAS PubMed.
- K. Hirota, Y. Nakayama and S. Nakane, US Pat., US 2010/0311561 A1, 2010.
- G. R. Anstis, P. Chantikul, B. R. Lawn and D. B. Marshall, J. Am. Ceram. Soc., 1981, 64, 533 CrossRef CAS PubMed.
- L. Y. Zheng, F. Z. Li and Y. C. Zhou, J. Am. Ceram. Soc., 2012, 95, 2028 CrossRef CAS PubMed.
- T. Wang and A. Yamaguchi, J. Mater. Sci. Lett., 2000, 19, 1245 Search PubMed.
- Y. Gao, Z. Huang, M. Fang, Y. Liu, S. Huang and X. Ouyang, Powder Technol., 2012, 226, 269 CrossRef CAS PubMed.
- V. V. Skorokhod, M. D. Vlajic and V. D. Krstic, Mater. Sci. Forum, 1998, 282–283, 219 CrossRef CAS.
- J. Deng and J. Sun, Ceram. Interfaces, 2009, 35, 771 CrossRef CAS PubMed.
- W. G. Fahrenholtz, E. W. Neuman, H. J. Brown-Shaklee and G. E. Hilmas, J. Am. Ceram. Soc., 2010, 93, 3580 CrossRef CAS PubMed.
- J. Zou, S. Huang, K. Vanmeensel, G. Zhang, J. Vleugels and O. van der Biest, J. Am. Ceram. Soc., 2013, 96, 1055 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |