A glowing antioxidant from tasar silk cocoon†
Abstract
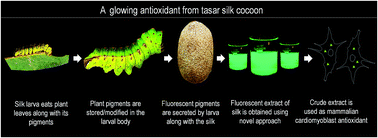
Oxidative stress is associated with a variety of disorders, diseases as well as the natural aging process thus making antioxidant discovery of medical relevance. In the present study, we report the simultaneous antioxidant as well as bio-imaging activities of a fluorescent extract (fluorophore) obtained from the tasar silk cocoons of the silk moth Antheraea mylitta. Using a nanocarrier-based strategy, we were able to localize the fluorophore in the cytosol of cardiomyoblast cell line H9c2 without any alterations to cellular morphology. In search of additional uses of the fluorophore beyond bio-imaging, we evaluated the antioxidant efficacies of the fluorophore against hydrogen peroxide-induced oxidative stress. Using microscopic, flow-cytometry and ELISA based studies; we observed that the silk fluorophore ameliorated hydrogen peroxide-induced reactive oxygen species (ROS) levels and oxidative stress. Moreover, enhanced levels of endogenous antioxidant enzymes were also observed in fluorophore pre-treated cells during oxidative stress. This resulted in significant reduction of oxidative stress-induced cell death. Our cumulative results suggest concomitant antioxidant and bio-imaging activities of the naturally occurring silk fluorophore. Given that a tasar silk fluorophore has never been isolated from cocoons in the silk textile industry, further commercial exploration will provide economic support to silk farmers.

 Please wait while we load your content...
Please wait while we load your content...