Synthesis of phenolic amides and evaluation of their antioxidant and anti-inflammatory activity in vitro and in vivo
Abstract
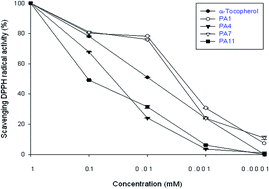
A series of 15 phenolic amides (PAs) have been synthesized (PA1–PA15) and examined in vitro by four different tests: (1) prevention of Cu2+-induced human low-density lipoprotein oxidation, (2) scavenging of stable radicals, (3) anti-inflammatory activity, (4) scavenging of superoxide radicals. We used PA1 and α-tocopherol for an in vivo study. The overall potential of the antioxidant system was significantly enhanced by the PA1 and α-tocopherol supplements as the hepatic TBARS levels were lowered while the hepatic SOD activities and GSH concentration were elevated in PA1 fed rats. Our results support that PA1 may exert antioxidant action through inhibiting superoxide generation. PA1 decreased the level of nitric oxide (NO) production, tumor necrosis factor-alpha (TNF-α) and nuclear factor-kappa B (NF-κB). These results show that PA1 can inhibit lipid peroxidation, enhance the activities of antioxidant enzymes, and decrease the TNF-α/NF-κB level and nitric oxide production. Therefore, it was speculated that PA1 acts through its anti-inflammation capacity.

 Please wait while we load your content...
Please wait while we load your content...