Synthesis of 1,5-dioxocanes via the two-fold C–O bond forming nucleophilic 4 + 4-cyclodimerization of cycloprop-2-en-1-ylmethanols†
Andrew Edwardsa,
Trevor Benninab,
Marina Rubinaa and
Michael Rubin*ac
aDepartment of Chemistry, University of Kansas, 1251, Wescoe Hall Dr., Lawrence, KS 66045-7582, USA. E-mail: mrubin@ku.edu
bDepartment of Chemistry, Northland College, 1411 Ellis Avenue, Ashland, Wisconsin 54806-3999, USA
cDepartment of Chemistry, North Caucasus Federal University, 1a Pushkin St., Stavropol 355009, Russia
First published on 19th August 2015
Abstract
An efficient [4 + 4] cyclodimerization of cyclopropenemethanols operating via a two-fold strain release-driven addition of alkoxides across the double bond of cyclopropenes was investigated. This chemo- and diastereoselective transformation provided previously unknown 2,7-dioxatricyclo[7.1.0.04,6]decane scaffolds.
Transition metal-catalyzed1 or photo-assisted2 4 + 4-cyclodimerizations with the simultaneous formation of two new C–C bonds are routinely used for assembly of eight-membered alicyclic compounds. However, analogous C–O bond-forming dimerization strategies for the preparation of eight-membered oxygen-based heterocycles remain much less explored. Nucleophilic closure of medium size rings is generally much more challenging than their five- and six-membered analogs due to a notable increase in ring strain (unfavorable enthalpic factor) and the accompanying significant loss of conformational freedom (unfavorable entropic factor). One of the few successful reported examples is the cyclodimerization of 2-alkoxyoxetanes, proceeding via acid-catalyzed or photo-induced reacetalization of ketals,3 ortho-esters,4 or enol ethers5 (Scheme 1, eqn (1)). Also, the assembly of eight-membered cyclic diesters via a double-fold Yamaguchi esterification of 3-hydroxypropanoic acids was employed during the total synthesis of (+)-bourgeanic lactone (eqn (2)).6 Somewhat less successful esterification under Steglich conditions providing cyclic trimers as major products was also reported.7 While the above examples involved carbonyl derivatives, synthesis of the 1,5-dioxocane core via a 4 + 4-cyclodimerization accompanied by the installation of two ethereal C–O bonds has not been reported to date. Herein we demonstrate an efficient and selective formation of the 1,5-dioxocane core via a strain release-driven double-fold addition of alkoxides across the double bond of cyclopropenes 3, providing access to peculiar 2,7-dioxatricyclo[7.1.0.04,6] decanes 4 (eqn (3)).
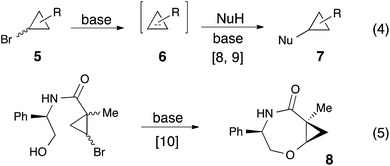
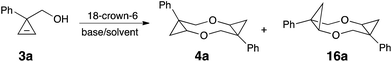
In our previous work on the development of practical synthetic approaches to cyclopropyl ether8 and cyclopropyl amine9 derivatives 7 via the formal nucleophilic substitution of cyclopropylhalides 5 (Scheme 2, eqn (4)),8,9 we have shown that a variety of alkoxides, and amides can be added across the double bond of in situ generated cyclopropenes. It was also demonstrated that an intramolecular version of this reaction could efficiently provide 2-oxabicyclo[5.1.0]octanes 8 (Scheme 2, eqn (5)).10 In our efforts toward expanding the scope of available strained substrates, we probed the reaction of (1-phenylcycloprop-2-en-1-yl)methanol (3a) under our standard reaction conditions with t-BuOK in THF in the presence of catalytic amount of 18-crown-6 ether (Table 1, entry 2).8 Remarkably, homodimerization outcompeted addition of the external nucleophile (t-BuO−) providing a single isomer of eight-membered cyclic ether 4a in 70% NMR yield and dr of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as the only observable product. Optimization studies11 proved powdered KOH to be a more efficient base than t-BuOK (Table 1, entry 1). It was also found that polar, aprotic, coordinating solvents were detrimental to the diastereoselectivity (Table 1, entries 5–13). Reactions performed in diethyl ether and toluene were selective, but much less efficient (entries 14–17). No product was observed in dichloromethane (entries 18 and 19), carbon tetrachloride (entries 20 and 21), and 1,4-dioxane (entries 22 and 23).
1, as the only observable product. Optimization studies11 proved powdered KOH to be a more efficient base than t-BuOK (Table 1, entry 1). It was also found that polar, aprotic, coordinating solvents were detrimental to the diastereoselectivity (Table 1, entries 5–13). Reactions performed in diethyl ether and toluene were selective, but much less efficient (entries 14–17). No product was observed in dichloromethane (entries 18 and 19), carbon tetrachloride (entries 20 and 21), and 1,4-dioxane (entries 22 and 23).
| # | Base (mass, mg) | Solvent (volume, mL) | Yielda, % | dr (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16a)b 16a)b |
|---|---|---|---|---|
| a NMR yields. Test reactions were performed in 6.3 mg (43 μmol) mmol scale (based on 3a) at 55 °C.11b Determined by GC analyses of crude reaction mixtures. | ||||
| 1 | KOH (12) | THF (1) | 74 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | t-BuOK (24) | THF (1) | 70 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | KOH (12) | THF (3) | 0 | — |
| 4 | t-BuOK (24) | THF (3) | 0 | — |
| 5 | KOH (12) | DMSO (1) | 32 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 6 | t-BuOK (24) | DMSO (1) | 75 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 7 | KOH (12) | DMSO (3) | 57 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 8 | t-BuOK (24) | DMSO (3) | 78 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 9 | t-BuOK (24) | DMSO (0.5) | 57 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 10 | KOH (12) | DMF (1) | 36 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 11 | t-BuOK (24) | DMF (1) | 57 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 12 | KOH (12) | DMA (1) | 36 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 13 | t-BuOK (24) | DMA (1) | 68 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 14 | KOH (12) | Et2O (1) | 60 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | t-BuOK (24) | Et2O (1) | 58 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 16 | KOH (12) | PhMe (1) | 28 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | t-BuOK (24) | PhMe (1) | 43 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 18 | KOH (12) | CH2Cl2 (1) | 0 | — |
| 19 | t-BuOK (24) | CH2Cl2 (1) | 0 | — |
| 20 | KOH (12) | CCl4 (1) | 0 | — |
| 21 | t-BuOK (24) | CCl4 (1) | 0 | — |
| 22 | KOH (12) | 1,4-Dioxane (1) | 0 | — |
| 23 | t-BuOK (24) | 1,4-Dioxane (1) | 0 | — |
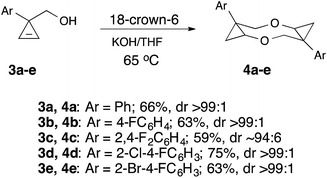
With the optimized procedure in hand we carried out preparative synthesis of 4a and its 4-fluoro- (3b), 2,4-difluoro- (3c), 2-chloro-4-fluoro- (3d), and 2-bromo-4-fluoro- (3e) substituted analogs 4b–e, all of which were obtained in good yields (Scheme 3).
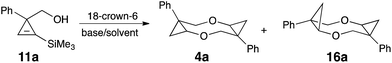
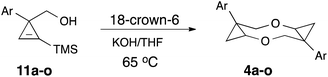
The starting cyclopropene alcohols 3 are readily available by reduction of the corresponding 1-arylcycloprop-2-ene-1-carboxylates 10 (Scheme 4),12 which are routinely obtained by Rh-catalyzed [2 + 1] cycloaddition of diazoarylacetates to trimethylsilylacetylene, followed by desilylation of the corresponding silylcyclopropenes 9.13 Alternatively, the reduction and the protodesilylation step can be swapped,14 which usually provides better yields in DIBAL reduction (9 → 11), but at the expense of efficiency on desilylation step (11 → 3) (Scheme 4). We proposed that desilylation and subsequent nucleophilic addition (3 → 4) could be combined in a one-pot sequence to obtain 1,5-dioxocanes directly from TMS-protected precursor 11. To test this idea, alcohol 11a (Ar = Ph) was subjected to the reaction conditions for 4 + 4-cyclodimerization described above. Gratifyingly, the same dioxocane 4a formed as sole isolable product in comparable yield (Table 2). Optimization of the reaction conditions revealed similar trends as the described above initial screening (Table 1); however, this one-pot transformation proceeded somewhat slower, requiring 5.5 equiv. of base (Table 2, entries 1–4) and slightly elevated temperature (entries 4–8) to achieve complete conversion.
| # | Base (mass, mg) | Solvent (1 mL) | Temp, °C (time, h) | Yielda, % | dr (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16a) 16a) |
|---|---|---|---|---|---|
| a NMR yields are listed.b Incomplete conversion: GC analysis showed presence of unreacted starting material 11a. Test reactions were performed in 9.3 mg (43 μmol) mmol scale based on 11a.11 | |||||
| 1 | KOH (4.5) | THF | 65 (24) | 72 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | KOH (9.5) | THF | 65 (24) | 68 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 3 | KOH (12) | THF | 65 (24) | 77 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 4 | KOH (24) | THF | 65 (24) | 73 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | KOH (12) | THF | 75 (24) | 49 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 6 | KOH (12) | THF | 55 (24) | 56 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 7 | KOH (12) | THF | 45 (24) | 21 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 8 | KOH (12) | THF | 35 (24) | 0 | — |
| 9 | t-BuOK (24) | THF | 65 (24) | 70 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | KOH (12) | DMSO | 65 (24) | 45 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 11 | t-BuOK (24) | DMSO | 65 (24) | 67 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 12 | KOH (12) | DMF | 65 (24) | 29b | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 13 | t-BuOK (24) | DMF | 65 (24) | 29b | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 14 | KOH (12) | DMF | 65 (72) | 34b | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 15 | t-BuOK (24) | DMF | 65 (72) | 21b | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 16 | KOH (12) | DMA | 65 (72) | 54 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 17 | t-BuOK (24) | DMA | 65 (72) | 48 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 18 | KOH (12) | Et2O | 65 (24) | 59 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 19 | t-BuOK (24) | Et2O | 65 (24) | 61 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 20 | KOH (12) | PhMe | 65 (72) | 71 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 21 | t-BuOK (24) | PhMe | 65 (72) | 75 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
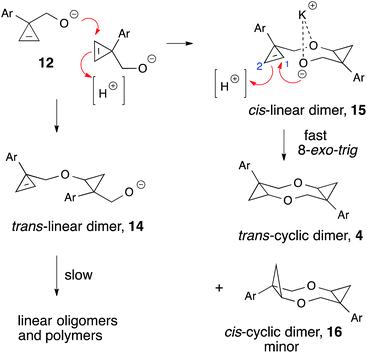
With more easily accessed, silyl-protected alcohols 11, we tested this reaction on fifteen other (1-aryl-2-silyl-cycloprop-2-en-1-yl)methanols possessing differently substituted aryl groups (Table 3). The first five examples shown in Table 3 (entries 1–5) allow for direct comparison of the one-pot desilylation/dimerization approach with the described above stepwise protocol (Scheme 3). In all cases the efficiency of the processes remained essentially the same. In the reactions of cyclopropenes 11c and 11j bearing two fluorine substituents competitive formation of two products was observed. These compounds were identified as diastereomers with trans- (1R*,4R*,6S*,9S*) (for major component) and cis- (1R*,4S*,6R*, 9S*) configurations, respectively. For all other examples the only isolable product was trans-2,7-dioxatricyclo[7.1.0.04,6]decane 4, which was unambiguously confirmed by single crystal X-ray crystallography of para-tolyl-substituted dioxocane 4g (Fig. 1, CCDC #1408273†). The high trans-selectivity observed in the formation of these rigid tricyclic products can be rationalized as follows (Scheme 5). Initially, the intermolecular nucleophilic attack8 of the primary alkoxide moiety in 12 at the double bond of the second cyclopropene molecule can potentially provide two intermediates: trans- (14) or cis-linear dimer (15), respectively. This strain release-driven step is highly exothermic and, therefore, essentially irreversible. Accordingly, the facial selectivity of this addition (in this case in the absence of efficient directing groups) should be exclusively governed by steric factors.8 The considerably larger size of the aryl substituent as compared to hydroxymethyl group is, therefore, the main reason for cis-diastereomer 15 to be formed predominantly. The preference of the second nucleophilic attack at diastereotopic C-1 vs. C-2 in the cyclopropene moiety of 15 leads to the highly selective formation of trans-cyclic dimer 4 with traces of cis-dimer 16 observed. Reasons affecting the stereo differentiation in the intramolecular 8-exo-trig cyclization at this point are not completely understood. It is believed that the initial pre-coordination of the alkoxide moiety with potassium cation affords a more favorable transition state leading to C2-symmetric product 4. This hypothesis is supported by experiments carried out in coordinating aprotic solvents (DMSO, DMF, DMA), which provided notably lower diastereoselectivities (Tables 1 and 2). Computational investigations that could support or rule out this hypothesis are currently underway in our laboratories and will be reported in due course. It should be mentioned that arguments pertaining to greater thermodynamic stability of cyclic dimer 4 vs. 16, can be ruled out since both steps (15 → 4) and (15 → 16) are irreversible (Scheme 5). Thus, our experiment showed that a sample of 4a generated in DMSO and partially enriched with cis-cyclic dimer 16a (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16a = 71
16a = 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29), being re-subjected to the reaction conditions did not change its composition.
29), being re-subjected to the reaction conditions did not change its composition.
| # | 11 | Ar | 4 | Yielda, % (dr) |
|---|---|---|---|---|
a Isolated yields of purified products are provided. Diastereomeric ratios were determined by GC analyses of crude reaction mixtures. Notation >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 indicates that minor diastereomer was below the detection limit.b Diastereomeric ratios were determined by 1H NMR of crude reaction mixtures. 1 indicates that minor diastereomer was below the detection limit.b Diastereomeric ratios were determined by 1H NMR of crude reaction mixtures. |
||||
| 1 | 11a | Ph | 4a | 64 (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
| 2 | 11b | 4-FC6H4 | 4b | 59 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 3 | 11c | 2,4-F2C6H3 | 4c | 62 (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)b 8)b |
| 4 | 11d | 2-Cl-4-FC6H3 | 4d | 79 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 5 | 11e | 2-Br-4-FC6H3 | 4e | 63 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 6 | 11f | 1-Naphthyl | 4f | 55 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 7 | 11g | 4-MeC6H4 | 4g | 78 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 8 | 11h | 2-Cl-6-FC6H3 | 4h | 69 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 9 | 11i | 2-ClC6H4 | 4i | 62 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 11 | 11j | 2,3-F2C6H3 | 4j | 57 (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12)b 12)b |
| 12 | 11k | 3-BrC6H4 | 4k | 83 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 13 | 11l | 4-BrC6H4 | 4l | 67 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 14 | 11m | 2,4-Cl2C6H3 | 4m | 65 (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 15 | 11n | 3-CF3C6H4 | 4n | 70 (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 16 | 11o | 2-Cl-4,5-F2C6H2 | 4o | 32 (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
 | ||
| Fig. 1 ORTEP drawing of 2,7-dioxatricyclo[7.1.0.04,6] decane 4g showing 50% probability amplitude displacement ellipsoids. | ||
It is also important to mention that the fate of the minor linear intermediate, trans-14 is completely different from that of cis-15, as it cannot undergo analogous cyclization. The cyclopropene and the alkoxymethyl moieties in trans-14 are located away from each other on the opposite sides of the cyclopropyl ring and, as a result, an intermolecular nucleophilic attack takes place predominantly, leading to linear oligomers and polymers. This bimolecular process is much slower, and allows for accumulation of intermediate 14 at initial stages of the reaction. By carrying out the reaction at slightly lower temperature, we were able to isolate 14a (Ar = Ph) in low yield (9%) and confirm its structure by spectral methods. Being re-subjected to the typical reaction conditions, 14a did not provide any cyclic products, but slowly polymerized instead. Polymerization of the alternate dimeric intermediate 14 under the reaction conditions significantly simplified isolation and purification of the tricyclic products 4, as upon completion of the reaction the crude mixture contained only one chromatographically mobile component accompanied by small amounts of immobile polymers.
Conclusions
In conclusion, we have demonstrated an efficient 4 + 4-cyclodimerization of (cycloprop-2-en-1-yl)methanols allowing for a single step assembly of medium sized cyclic ethers via the simultaneous formation of two ethereal C–O bonds. The described base-assisted, strain release-driven transformation proceeds via a sterically-controlled, facially-selective, intermolecular nucleophilic addition of alkoxides across the double bond of cyclopropenes followed by a diastereoselective ring closure, furnishing an unusual 2,7-dioxatricyclo[7.1.0.04,6]decane core. To the best of our knowledge, this is the first example of a 4 + 4-cyclodimerization involving nucleophilic addition of oxygen-based nucleophiles to olefin moieties. Sterically controlled facial selectivity of the intermolecular attack in the first step of the reaction translates into the high chemoselectivity of the subsequent intramolecular cyclization. Such “natural selection”, in which only the major intermediate, cis-linear dimer can participate in cyclization, while the minor trans-linear dimer polymerizes, results in the C2-symmetric tricyclic compounds obtained exclusively in good yields and with excellent diastereoselectivities.Acknowledgements
Financial support from International Collaboration Program, supported by the Ministry of Education and Science of the Russian Federation and the Ministry of Education of Perm Krai is gratefully acknowledged. We also are grateful for support by the Russian Fund for Basic Research (grant #15-03-02661) and NSF REU program grant #CHE-1263259 for student support (TB). Support for the NMR instrumentation was provided by NIH Shared Instrumentation Grant #S10RR024664 and NSF Major Research Instrumentation Grant #0320648.Notes and references
- For review, see: (a) J. Montgomery, Sci. Synth., 2002, 1, 11 CAS ; see also: ; (b) R. Shintani, T. Tsuji, S. Park and T. Hayashi, Chem. Commun., 2010, 46, 1697 RSC; (c) P. H. Lee, K. Lee and Y. Kang, J. Am. Chem. Soc., 2006, 128, 1139 CrossRef CAS PubMed; (d) P. H. Lee and K. Lee, Angew. Chem., Int. Ed., 2005, 44, 3253 CrossRef CAS PubMed; (e) M. A. ElAmrani, I. Suisse, N. Knouzi and A. Mortreux, Tetrahedron Lett., 1995, 36, 5011 CrossRef CAS; (f) A. Bendayan, H. Masotti, G. Peiffer, C. Siv and A. Archavlis, J. Organomet. Chem., 1993, 444, 41 CrossRef CAS; (g) K. Masuda, K. Nakano, T. Fukahori, H. Nagashima and K. Itoh, J. Organomet. Chem., 1992, 428, C21 CrossRef CAS; (h) K. U. Baldenius, H. T. Dieck, W. A. Koenig, D. Icheln and T. Runge, Angew. Chem., Int. Ed., 1992, 31, 305 CrossRef PubMed.
- (a) M. V. S. N. Maddipatla, M. Pattabiraman, A. Natarajan, K. Srivastav, J. T. Mague and V. Ramamurthy, Org. Biomol. Chem., 2012, 10, 9219 RSC; (b) A. Michaelides, S. Skoulika and M. G. Siskos, Chem. Commun., 2011, 47, 7140 RSC.
- V. A. Petrov and W. Marshall, Beilstein J. Org. Chem., 2010, 6(46) DOI:10.3762/bjoc.6.46.
- N. J. Turro and J. R. Williams, Tetrahedron Lett., 1969, 10, 321 CrossRef.
- T. Machiguchi, J. Okamoto, Y. Morita, T. Hagesawa, S. Yamabe and T. Minato, J. Am. Chem. Soc., 2006, 128, 44 CrossRef CAS PubMed.
- (a) J. S. Yadav, K. V. R. Rao, K. Ravindar and B. V. S. Reddy, Eur. J. Org. Chem., 2011, 58 CrossRef CAS PubMed; (b) J. S. Yadav, T. S. Rao, N. N. Yadav, K. V. R. Rao, B. V. S. Reddy and A. A. K. Al Ghamdi, Synthesis, 2012, 44, 788 CrossRef CAS.
- H. J. Rogers, J. Chem. Soc., Perkin Trans. 1, 1995, 3073 RSC.
- (a) P. Ryabchuk, A. Edwards, N. Gerasimchuk, M. Rubina and M. Rubin, Org. Lett., 2013, 15, 6010 CrossRef CAS PubMed; (b) J. E. Banning, A. R. Prosser, B. K. Alnasleh, J. Smarker, M. Rubina and M. Rubin, J. Org. Chem., 2011, 76, 3968 CrossRef CAS PubMed; (c) J. E. Banning, A. R. Prosser and M. Rubin, Org. Lett., 2010, 12, 1488 CrossRef CAS PubMed.
- (a) J. E. Banning, J. Gentillon, P. G. Ryabchuk, A. R. Prosser, A. Rogers, A. Edwards, A. Holtzen, I. A. Babkov, M. Rubina and M. Rubin, J. Org. Chem., 2013, 78, 7601 CrossRef CAS PubMed; (b) P. Ryabchuk, M. Rubina, J. Xu and M. Rubin, Org. Lett., 2012, 14, 1752 CrossRef CAS PubMed; (c) A. R. Prosser, J. E. Banning, M. Rubina and M. Rubin, Org. Lett., 2010, 12, 3968 CrossRef CAS PubMed.
- B. K. Alnasleh, W. M. Sherrill, M. Rubina, J. Banning and M. Rubin, J. Am. Chem. Soc., 2009, 131, 6906 CrossRef CAS PubMed.
- See ESI†.
- M. Rubina, M. Rubin and V. Gevorgyan, J. Am. Chem. Soc., 2004, 126, 3688 CrossRef CAS PubMed.
- A. Edwards and M. Rubin, Tetrahedron, 2015, 71, 3237 CrossRef CAS PubMed.
- X. Liu and J. M. Fox, J. Am. Chem. Soc., 2006, 128, 5600 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. CCDC 1408273. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14077c |
| This journal is © The Royal Society of Chemistry 2015 |