A colorimetric/luminescent benzene compound sensor based on a bis(σ-acetylide) platinum(ii) complex: enhancing selectivity and reversibility through dual-recognition sites strategy†
Abstract
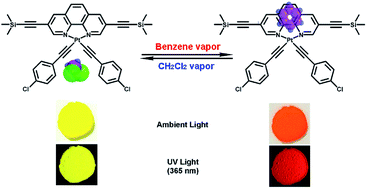
A square-planar bis(σ-acetylide) Pt(II) complex containing dual-recognition sites was designed, synthesized and used as a colorimetric/luminescent sensor for detecting the vapor of benzene compounds.

 Please wait while we load your content...
Please wait while we load your content...