l-Asparagine-assisted synthesis of flower-like β-Bi2O3 and its photocatalytic performance for the degradation of 4-phenylphenol under visible-light irradiation†
Abstract
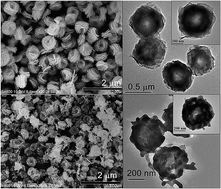
Large-scale synthesis of nanosized β-Bi2O3 is a significant challenge due to its metastable state. A facile L-asparagine-assisted reflux–calcination route was successfully developed for the large-scale preparation of β-Bi2O3 micro/nanostructures under mild conditions (low temperature, atmospheric pressure, and wide temperature windows). The composition, phase structure, morphology, surface area, and photoabsorption properties of as-synthesized β-Bi2O3 and its precursor were systematically characterized. The phase transformation conditions and possible formation mechanism of flower-like β-Bi2O3 were discussed. It is found that with a simple reflux process under atmospheric pressure at 100 °C, uniform monodisperse bismuth–asparagine complex microspheres with average diameters of ∼500 nm were produced and flower-like β-Bi2O3 micro/nanostructures were then conveniently obtained after precursor calcination at temperatures ranging from 340 °C to 420 °C. A surface CO32− coordination effect introduced from L-asparagine explained the formation of stabilized β-Bi2O3 at low temperatures (up to 420 °C). The as-synthesized β-Bi2O3 shows excellent photocatalytic activity toward the degradation of 4-phenylphenol under visible-light irradiation, which is 3.7 and 21.4 times faster than the removal rates of β-Bi2O3 nanospheres and a commercial β-Bi2O3, respectively, and allows for the elimination of 93.2% total organic carbon after 60 min of irradiation. In addition, the photogenerated reactive species were identified by radical scavenger experiments and electron paramagnetic resonance spectroscopy, and a possible visible-light-induced photocatalytic mechanism was then proposed.

 Please wait while we load your content...
Please wait while we load your content...