Formation of lipid and polymer based gold nanohybrids using a nanoreactor approach†
Dominik Witzigmanna,
Sandro Siebera,
Fabiola Portaa,
Philip Grossena,
Andrej Bierib,
Natalja Strelnikovac,
Thomas Pfohlc,
Cristina Prescianotto-Baschongd and
Jörg Huwyler*a
aDivision of Pharmaceutical Technology, Department of Pharmaceutical Sciences, University of Basel, Klingelbergstrasse 50, Basel CH-4056, Switzerland. E-mail: joerg.huwyler@unibas.ch
bCenter for Cellular Imaging and NanoAnalytics (C-CINA), Biozentrum, University of Basel, Mattenstrasse 26, Basel CH-4058, Switzerland
cDepartment of Chemistry, University of Basel, Klingelbergstrasse 80, Basel CH-4056, Switzerland
dBiozentrum, University of Basel, Klingelbergstrasse 70, Basel CH-4056, Switzerland
First published on 25th August 2015
Abstract
Nanocarriers encapsulating gold nanoparticles (AuNPs) hold tremendous promise for numerous biomedical applications. So far only a few fabrication strategies have been investigated and efficient processes for the manufacturing of gold nanohybrids (AuNHybs) are still missing. We encapsulated a tetrachloroaurate/citrate mixture within nanocarriers and initiated the AuNP formation after self-assembly of the nanomaterial by a temperature shift. This nanoreactor approach was successfully combined with the film-rehydration, nanoprecipitation, or microfluidics method. Different nanomaterials were validated including phospholipids and copolymers and the process was optimized towards encapsulation efficiency and physico-chemical homogeneity of AuNHybs. Our nanoreactor technology is versatile, efficient, and highly reproducible. Dynamic light scattering and electron microscopy techniques confirmed that generated lipid and polymer based AuNHybs were of uniform size below 130 nm and contained a single AuNP. The AuNHyb solutions had a deep-red color and exhibited the specific surface plasmon absorption of AuNPs. The unique optical properties of AuNHybs were used to visualize cellular uptake of nanocarriers in vitro demonstrating the promising applicability of AuNHybs as a bioimaging tool.
1. Introduction
Gold nanoparticles (AuNPs) have attracted great interest since Michael Faraday first described them in 1857.1,2 The application of AuNPs in the field of imaging and therapy were based on their unique properties, which include; (I) advantageous physico-chemical characteristics, (II) non-toxic and inert properties, (III) facile preparation of monodisperse AuNPs, and (IV) various modification options.3–5 Different methods for the synthesis of AuNPs have been described.6,7 The most widely used approach is the chemical reduction of gold salt (Au3+) such as tetrachloroaurate (HAuCl4) to metallic gold (Au0) using the Turkevich8,9 or Brust-Schiffrin10 method. Moreover, synthesis methods using microwaves, UV irradiation, microfluidics, or biologic approaches were examined.3,11 Ultimately, AuNPs have to be modified with capping agents to avoid aggregation, provide solubility in aqueous media, and improve stability.12Encapsulation of AuNPs into different nanocarriers such as liposomes or polymeric nanoparticles is an interesting option for a wide range of applications such as smart drug delivery, imaging, or photothermal therapy.13–18 In recent decades, great progress has been made in the field of hybrid nanocarriers using AuNPs and several strategies for their synthesis have been investigated. For example, lipid based gold nanohybrids (lipid-AuNHybs) have been prepared using the following methods: improved cholate dialysis,19 incorporation of hydrophobic AuNPs,20–22 physical absorption,23,24 or precipitation of gold within liposomes using either, glycerol including formation or reverse-phase evaporation.25,26
However, these strategies exhibited marked variability in homogeneity, reproducibility, size distribution, and morphology of gold nanohybrids (AuNHybs). To overcome these challenges, we developed a novel and versatile strategy to encapsulate AuNPs into different nanocarriers with high reproducibility using a nanoreactor approach. The goal of the present study was, (I) the encapsulation of a tetrachloroaurate/citrate mixture within nanocarriers and (II) the initiation of AuNP formation after self-assembly of the nanomaterial. Selected nanomaterials (i.e. lipid and polymer based) were validated and the encapsulation efficiency, homogeneity, and robustness of our approach were optimized. Nanocarriers loaded with AuNPs were prepared by three different methods depending on the physico-chemical properties of the nanocarrier material.
The film-rehydration–extrusion method27 was used for conventional (non-PEGylated) liposomes, the nanoprecipitation method28 was used for di-block copolymer nanoparticles, and the microfluidics method29,30 was used for PEGylated (sterically stabilized) liposomes (Fig. 1). The most important feature of our nanoreactor approach is the production of nanocarriers at room temperature (RT), which avoids the formation of AuNPs before self-assembly. AuNP formation is subsequently initiated by a temperature shift. The applicability of the AuNHybs as bioimaging tool was demonstrated in vitro using HepG2 human hepatocellular carcinoma cells.
2. Experimental section
2.1 AuNP synthesis
AuNPs were synthesized following a modified Turkevich method.7 Optimization of AuNP synthesis using a 23 full factorial design of experiment (DoE) [Stavex 5.2, Aicos Technologies, Basel, Switzerland] is described in detail in the ESI (Table S1†). Briefly, ddH2O with 1 mM tetrachloroaurate (Sigma-Aldrich, Buchs, Switzerland) were heated to 70 °C for 20 min under vigorous stirring. To start the formation of AuNPs, citrate solution (170 mM; 50 mg mL−1) was added as a reducing and capping reagent. The HAuCl4/citrate gold reaction mixture (AuR-solution) was stirred at 70 °C for 10 min until the solution had a deep-red color.2.2 AuNHyb formation using film rehydration
The film rehydration method was used for lipids with a transition temperature (Tm) below RT with modifications described elsewhere.31 Liposomes were produced at a temperature which inhibits the AuNP formation. In brief, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (15 μmol) [POPC] (Avanti Polar-Lipids, Alabaster, USA) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-1′-rac-glycerol (5 μmol) [POPG] (Avanti Polar-Lipids, Alabaster, USA) were dissolved in chloroform/methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and a homogenous dry lipid film was prepared using a Rotavapor A-134 (Büchi, Flawil, Switzerland). The lipid film was rehydrated with a freshly prepared AuR-solution (HAuCl4
1, v/v) and a homogenous dry lipid film was prepared using a Rotavapor A-134 (Büchi, Flawil, Switzerland). The lipid film was rehydrated with a freshly prepared AuR-solution (HAuCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citrate ratio – 1
citrate ratio – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) at RT and 120 rpm for 10 min with 3 g glass beads (diameter 5 mm). Different lipid (20 mM) to AuR-solution ratios were tested starting from 1 mM HAuCl4/4.1 mM citrate up to 8 mM HAuCl4/32.8 mM citrate.
4) at RT and 120 rpm for 10 min with 3 g glass beads (diameter 5 mm). Different lipid (20 mM) to AuR-solution ratios were tested starting from 1 mM HAuCl4/4.1 mM citrate up to 8 mM HAuCl4/32.8 mM citrate.
The resulting multilamellar vesicles were subjected to three freeze–thaw cycles and extruded through polycarbonate membranes with two different pore sizes using a barrel extruder (Lipex; Northern Lipids, Vancouver; Canada). Liposomes were extruded at RT 3 times through a 200 nm polycarbonate membrane and 11 times through a membrane with a pore size of 100 nm (VWR International, Dietikon, Switzerland). AuNPs, which were formed during the extrusion procedure, bind to the filter membranes. Finally, the unilamellar liposomes were heated to 70 °C for 10 min to start the formation of AuNPs. To separate liposomes from free AuNPs, the sample was purified by FPLC using a Superose 6 prep column (GE Healthcare, Glattbrugg, Switzerland) eluting with 0.01 M phosphate buffered saline (PBS) containing 150 mM sodium chloride, pH 7.4 (Sigma-Aldrich, Buchs, Switzerland).
2.3 AuNHyb formation using nanoprecipitation
A nanoprecipitation method was used for the di-block copolymer polyethyleneglycol–polycaprolactone [PEG–PCL] (Sigma-Aldrich, Buchs, Switzerland). The polymer (5 mg) was dissolved in THF (50 μL) [Sigma-Aldrich, Buchs, Switzerland] under constant stirring with a magnetic bar (750 rpm). The AuR-solution (HAuCl4 : citrate ratio – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was added dropwise (one drop per five seconds). The mixture was stirred for 10 min at 750 rpm followed by 10 min on a thermomixer at 70 °C and 300 rpm. Different polymer to AuR-solution ratios were tested starting from 1 mM HAuCl4/4.1 mM citrate up to 8 mM HAuCl4/32.8 mM citrate. To separate the PEG–PCL–AuNHybs from free AuNPs, a FPLC purification step was used (see above).
4) was added dropwise (one drop per five seconds). The mixture was stirred for 10 min at 750 rpm followed by 10 min on a thermomixer at 70 °C and 300 rpm. Different polymer to AuR-solution ratios were tested starting from 1 mM HAuCl4/4.1 mM citrate up to 8 mM HAuCl4/32.8 mM citrate. To separate the PEG–PCL–AuNHybs from free AuNPs, a FPLC purification step was used (see above).
2.4 Microfluidics device design and fabrication
The microfluidics device was fabricated with 0.05 mm thick polystyrene foil (GoodFellow, Huntingdon, UK) and NOA 81 (Norland, Cranberry, USA) using standard soft-lithography techniques according to the procedure described previously.32,33 The microfluidics device had seven inlet channels converging to a single staggered herringbone micromixer. The rectangular cross-section had dimensions of 468 μm length, 80 μm width, and 40 μm/200 μm height (Fig. 1C). Detailed experimental procedures are given in the ESI.†2.5 Flow visualization and computational fluid dynamics simulation
Detailed experimental procedures are given in the ESI.†2.6 AuNHyb formation using microfluidics
The microfluidics method was used for lipids with a transition temperature above RT (55 °C). Therefore, the AuNP formation during the liposome production was hampered due to decreased temperature. 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) [5.75 μmol] (Avanti Polar-Lipids, Alabaster, USA), cholesterol [4 μmol] (Sigma-Aldrich, Buchs, Switzerland), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy(polyethylene glycol)-2000) (DSPE-PEG2000) [0.25 μmol] (Avanti Polar-Lipids, Alabaster, USA) were dissolved in ethanol. The microfluidics device was primed with water for the outer streams and with ethanol for the central inlet (1 μL s−1) using syringe pumps.Afterwards four different syringes were connected: (I) double-distilled water, (II) citrate solution (4.1 mM–32.8 mM), and (III) tetrachloroaurate (HAuCl4) solution (1 mM–8 mM) were connected to the outer streams (always with a HAuCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citrate ratio of 1
citrate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and (IV) lipids in ethanol (5 mM) were connected to the central inlet. The speed was set to 4 μL s−1 for syringe I, 2 μL s−1 for syringe II/III and 1 μL s−1 for syringe IV. The sample was collected at the outlet. Finally, the liposomes were heated to 70 °C for 10 min to start the formation of AuNPs. To separate lipid-AuNHybs from free AuNPs, the sample was purified by FPLC using a Superose 6 prep column eluting with 0.01 M PBS pH 7.4.
4) and (IV) lipids in ethanol (5 mM) were connected to the central inlet. The speed was set to 4 μL s−1 for syringe I, 2 μL s−1 for syringe II/III and 1 μL s−1 for syringe IV. The sample was collected at the outlet. Finally, the liposomes were heated to 70 °C for 10 min to start the formation of AuNPs. To separate lipid-AuNHybs from free AuNPs, the sample was purified by FPLC using a Superose 6 prep column eluting with 0.01 M PBS pH 7.4.
2.7 Size analysis using dynamic light scattering
Dynamic light scattering (DLS) measurements of AuNPs and all lipid- and polymer-AuNHybs were conducted using a Delsa Nano C Particle Analyzer (Beckman Coulter, Nyon, Switzerland). The laser was adjusted to 658 nm and scattered light was detected at a 165° angle. Data was converted using CONTIN particle size distribution analysis. The AuNHyb size was analyzed three times in PBS at RT.2.8 UV-Vis and fluorescence measurements
Ultraviolet-visible (UV-Vis) absorption from 260 nm to 750 nm (step size one nm) of different samples was measured using a SpectraMax M2 (Molecular Devices, Sunnyvale, USA). Fluorescence of lipid-AuNHybs containing rhodamine labelled phospholipids (Rho-PE) [Avanti Polar-Lipids, Alabaster, USA] was analyzed by excitation at 560 nm and detection between 572 nm to 750 nm.2.9 Transmission electron microscopy of gold nanohybrids
Size and shape of the AuNPs and AuNHybs were analyzed by transmission electron microscopy (TEM) using a CM-100 (Philips, Eindhoven, Netherlands) operating at 80 kV. Samples were prepared by deposition onto a 400-mesh carbon-coated copper grid (Polysciences Inc., Eppelheim, Germany). Prior to sample deposition, the grid was exposed to plasma for 10 seconds to increase sample binding. Grids were washed with double-distilled water to prevent precipitation of uranyl salts by phosphate ions. Then the samples were negatively stained using a 2% uranylacetate solution (Sigma-Aldrich, Buchs, Switzerland), the excess of uranylacetate was removed using filter paper, and the samples were dried at RT overnight. Nanocarrier integrity was preserved by this procedure as confirmed by Cryo-EM analysis using sample vitrification (see below). To characterize the size of AuNPs inside of AuNHybs, the diameter of at least 100 AuNPs was determined.2.10 Cryo-TEM of gold nanohybrids
Aliquots (4 μL) of AuNHybs were adsorbed onto holey-carbon supported grids (Quantifoil, Glossloebichau, Germany), blotted with Whatman 1 filter papers, and vitrified in liquid nitrogen-cooled liquid ethane using a Vitrobot IV (FEI Company, Eindhoven, Netherland). Cryo-electron imaging was performed with a Philips CM200-FEG electron microscope operated at an acceleration voltage of 200 kV. Micrographs were recorded with a 4k × 4k TemCam-F416 CMOS camera (TVIPS, Gauting, Germany).2.11 Preparation of fluorescent lipid based AuNHybs
Detailed experimental procedures are given in the ESI.†2.12 Passive uptake of AuNPs and AuNHybs in HepG2 cells
HepG2 cells were seeded on a 10 cm plate and cultured in 10 mL Dulbecco's modified Eagle's culture medium high glucose supplemented with 10% fetal calf serum (FCS), 100 units mL−1 penicillin, and 100 μg mL−1 of streptomycin (DMEM comp). All cell culture media components were purchased from Sigma-Aldrich (Buchs, Switzerland). Cells were allowed to adhere for 24 h before the AuNPs or AuNHybs were added. After incubation at 37 °C for 18 h, the tissue culture plate was washed three times with DMEM comp (37 °C). Afterwards, the cells were fixed with DMEM containing 3% formaldehyde (Sigma-Aldrich, Buchs, Switzerland) and 0.3% glutaraldehyde (Sigma-Aldrich, Buchs, Switzerland) for two hours at RT and stored overnight at 4 °C. The following day, cells were scraped, pelleted, and washed three times with water, and then incubated with 2% uranylacetate for two hours at 4 °C in the dark. The sample was washed, dehydrated by series of methanol, and infiltrated with LR-gold resin (London Resin, London, UK) according to the manufacturer's instructions. Polymerization was performed at −10 °C by UV light for one day. Sections of about 70 nm were collected on carbon-coated Formvar-Ni-grids (EMS, Hatfield, USA) and stained for 15 min with 4% uranylacetate followed by two minutes in Reynolds lead citrate solution. Sections were viewed using a Phillips CM-100 electron microscope.3. Results and discussion
3.1 Film rehydration (conventional liposomes)
For the preparation of AuNP loaded liposomes, the most direct approach is the rehydration of a lipid film with presynthesized AuNPs. The well-characterized method developed by Turkevich and Frens is ideal to synthesize AuNPs with diameters of approximately 20 nm (see ESI† for experimental details).7,34,35 However, the AuNP encapsulation approach has several issues. The AuNPs often form aggregates up to several hundred nanometers (Fig. 2A), which results in low encapsulation efficiency (Fig. 2D) and renders extrusion impossible due to blocked filter membranes. Therefore, we developed an alternative strategy and combined the film-rehydration method with a ‘nanoreactor approach’. We rehydrated the lipid film with the Turkevich reaction mixture consisting of tetrachloroaurate and citrate. Then the formation of AuNPs was initiated inside the core of preassembled liposomes by a shift in temperature [70 °C, 10 min] (Fig. 1A).To prevent the formation of AuNPs during the preparation of liposomes, we selected lipids that are characterized by a low transition temperature (Tm). The lipid composition consisted of POPC and POPG, which provides a Tm of −2 °C. The lipid film was rehydrated with a tetrachloroaurate and citrate reaction solution in different ratios and the liposomes were extruded at RT before initiation of AuNP formation. The final AuNHyb sample exhibited the characteristic ruby-red color resulting from the surface plasmon resonance of encapsulated AuNPs.36 To test if the temperature affected the efficiency of the process, the entire procedure was also carried out at 4 °C. However, there was no difference between AuNHybs prepared at RT or at lower temperatures. TEM showed that the AuNPs were encapsulated inside the AuNHybs (Fig. 2B). Encapsulation efficiency (i.e. the ratio between liposomes encapsulating AuNPs and liposomes that were empty) was significantly higher using the nanoreactor approach compared with either extrusion at high temperature (data not shown) or use of preformed AuNPs (Fig. 2D). The latter condition lead to the formation of AuNP aggregates in the medium surrounding the liposomes (Fig. 2D). In contrast, the nanoreactor approach resulted in the encapsulation of a single AuNP in the inner liposomal core.
POPC/POPG–AuNHybs were also analyzed by Cryo-TEM (Fig. 2C). Under these conditions, the native, hydrated state of the lipid formulation is presented.37 Cryo-TEM showed that lipid-AuNHybs were spherical, mainly unilamellar, and efficiently loaded with AuNPs (Fig. 2C). AuNPs were located in the hydrophilic core of the liposomes (Fig. 2C), consistent with TEM analysis (Fig. 2B). Interestingly, some AuNPs were located in close proximity to the lipid bilayer. This could be either an artefact from the drying process during the TEM grid preparation or an interaction of the AuNPs with one of the phospholipids. The number of AuNPs encapsulated was dependent on the concentration of the AuR-solution. The highest AuNP encapsulation efficiency was achieved with 4 mM tetrachloroaurate, 16.3 mM citrate, and 20 mM lipids. Higher AuR-solution to lipid ratios resulted in the formation of AuNP agglomerates outside of the liposomes. On the other hand, lower AuR-solution to lipid ratios led to a significant amount of empty nanocarriers. Interestingly, TEM analysis revealed that the AuNPs, which were synthesized inside liposomes were significantly smaller than AuNPs synthesized without lipids (approximately 12.0 nm vs. 21.5 nm) (Fig. 2A vs. Fig. 2B). It is tempting to speculate that the limited amount of tetrachloroaurate and citrate available inside the nanocarriers is limiting the maximum size of the AuNPs. The POPC/POPG–AuNHybs were analyzed by DLS. The size of POPC/POPG–AuNHybs (104.7 nm ± 5.2 nm) was similar to the size of empty liposomes (Fig. 3A). Thus, AuNPs did not influence the nanocarrier diameter. In Fig. 4A, the absorption spectra of empty POPC/POPG liposomes (negative control), AuNPs (positive control), and POPC/POPG–AuNHybs are compared. As expected no absorption maximum was observed for empty POPC/POPG liposomes in the wavelength range from 500 nm to 600 nm. In contrast, AuNPs and POPC/POPG–AuNHybs showed a distinct surface plasmon absorbance peak at 525 nm. This indicates the successful encapsulation of AuNPs inside POPC/POPG liposomes.
3.2 Nanoprecipitation (di-block copolymer nanoparticles)
To demonstrate the broad applicability of our nanoreactor approach, we investigated the formation of polymer-AuNHybs using nanoprecipitation (Fig. 1B). For this method, the AuR-solution was added dropwise to the di-block copolymer PEG–PCL dissolved in THF (see ESI† for experimental details). Similar to the lipid-AuNHybs, the addition of preformed AuNPs to the polymer resulted in low encapsulation efficiency (Fig. S1A†). In contrast, high encapsulation efficiency with one AuNP per polymeric nanocarrier was achieved using the nanoreactor approach (Fig. 2E). Cryo-TEM analysis confirmed that AuNPs with a size of 14.1 nm ± 3.1 nm were located inside the polymer-AuNHybs (Fig. S1B†).Polymer-AuNHybs consisting of PEG–PCL presented a spherical morphology in which polymer chains self-assemble as solid polymeric nanoparticles. Thus, the hydrophilic parts of the di-block copolymer are exposed towards the outer buffer environment (Fig. S1B†). Recently, this was also shown for other polymeric nanoparticles.38 DLS analysis showed a monodisperse formulation of polymer-AuNHybs (polydispersity index = 0.088) with a diameter of 77.5 nm ± 3.9 nm (Fig. 3B). Additionally, the polymer-AuNHybs showed a characteristic surface plasmon band at 527 nm due to the unique optical properties of AuNPs (Fig. 4B).
3.3 Microfluidics (PEGylated liposomes)
Recently, it has been demonstrated that rapid microfluidic mixing offers a controlled method to produce lipid nanocarriers.39 Defined interfacial forces between the nanomaterial components result in a controllable and highly reproducible self-assembly process.40 To facilitate the production of AuNP loaded PEGylated liposomes, we developed a microfluidics platform for the preparation of lipid-AuNHybs using lipids with a transition temperature above RT. Briefly, PEGylated lipid based gold nanohybrids (PEG–Lipo–AuNHybs) were synthesized by rapid mixing of an ethanolic lipid solution (5 mM, consisting of DSPC, cholesterol, and DSPE-PEG2000) and the aqueous Turkevich AuR-solution. In the last step of the process, the PEGylated liposomes were heated to 70 °C to start the formation of the AuNPs inside the liposomes.In more detail, our microfluidics device consisted of seven inlet channels which converged into a single staggered-herringbone micromixer (see ESI†). At the junction of the inlets, the center stream was hydrodynamically focused to improve the mixing. To illustrate the nanomanufacturing process, we simulated the flow patterns and visualized them with a fluorescent dye (Fig. 1D/E; ESI Fig. S2†). Liposomes are formed at the interface between the hydrodynamically focused lipid stream and the AuR-solution. The mechanism of controlled, focused, and rapid mixing41 is visible both in the experimental setting (Fig. 1D) and the computer simulation (Fig. 1E). The central inlet was used for the model ethanolic lipid solution [5 mM] (Fig. S2B†). The other six, outer inlets were used for tetrachloroaurate (Fig. S2C†), citrate (Fig. S2D†), and water (two inlets for each solution). Syringe pumps were used with flow rates up to 4 μL s−1. The reagents were supplied by separate inlets because the use of tetrachloroaurate and citrate in the same inlet resulted in premature AuNP formation in the herringbone micromixer (Fig. S3D†).40 In addition, the use of preformed AuNPs resulted in a low encapsulation efficiency, as observed for the film-rehydration and nanoprecipitation method with preformed AuNPs (Fig. S3E†). Therefore, the best results were achieved using a microfluidics platform with seven different inlets (Fig. 1C–E).
Water (4 μL s−1) was used to improve the hydrodynamic flow focusing of the lipid stream and to decrease the final ethanol concentration. Furthermore, we used a higher flow rate for the outer inlets to increase the water to ethanol ratio. This is important to prevent the destabilization of liposomes caused by elevated ethanol concentrations.42 Microfluidics parameters were adjusted to optimize encapsulation efficiency and size distribution. A continuous and efficient production was achieved with 2 mM tetrachloroaurate/8.2 mM citrate solution (for 5 mM lipids); and flow rates of 2 μL s−1 for the AuR-solutions, and 1 μL s−1 for the lipid solution. The absolute production speed was 420 μL min−1 (Fig. S2A†). Increasing the concentration of the AuR-solution for the microfluidics manufacturing resulted in an increased number of encapsulated AuNPs per AuNHyb (Fig. S3A–C†) until agglomerates were observed. AuNHybs with several encapsulated AuNPs mostly resulted in Janus-like vesicle structures (i.e. asymmetrical loading) as recently shown for Janus magnetic liposomes.43 DLS and TEM showed that the size of PEG–Lipo–AuNHybs was similar to that of empty PEGylated liposomes (Fig. 2F and 3C). PEG–Lipo–AuNHybs with a mean hydrodynamic diameter of 123 nm ± 2.5 nm (Z-average) and a monodisperse size distribution were obtained (Fig. 3C). Each liposome incorporated one AuNP with a diameter of 6.8 nm ± 1.5 nm as shown by TEM (Fig. 2F). A characteristic plasmon absorption at 525 nm was observed using UV-Vis spectroscopy (Fig. 4C).
3.4 Characterization of nanoreactor approach
We showed that our nanoreactor approach is applicable for a wide range of nanomaterials, as well as different preparation methods. The film rehydration method can be used for lipids with a transition temperature below RT, whereas the nanoprecipitation method is suitable for several polymer nanomaterials.28,44 The microfluidics method is especially designed for lipids with a transition temperature above RT. However, this technique is suitable for all nanomaterials, that need highly controlled nanomanufacturing.40The most important step of our nanoreactor approach is the speed of the nanocarrier production. The nanocarrier formation needs to be faster than AuNP aggregate formation. Therefore, the AuNP formation inside the nanocarriers is initiated by a temperature shift after self-assembly. AuNP aggregates formed outside of the self-assembled nanocarrier are removed by size exclusion chromatography. During this step, the outer buffer can be exchanged to a physiological compatible medium such as PBS. Several publications have shown that a difference in tonicity between the nanocarrier lumen and the outer buffer environment is not affecting the stability or morphology of nanocarriers.45–47
All tested combinations resulted in efficient and reproducible formation of AuNHybs. Moreover, DLS, TEM, and Cryo-TEM analysis showed that the final AuNHybs preserved the initial dimensions of the empty nanocarriers. Four distinct observations indicate that the AuNPs were encapsulated inside the nanocarriers. Firstly, the AuNHybs passed easily through the size exclusion chromatography medium (Superose 6 prep) whereas non-encapsulated AuNPs showed a high affinity for the chromatography medium, which was recently also shown for quantum dots.48 Secondly, the AuNHybs exhibited an improved stability in PBS and aggregation was prevented as compared to free AuNPs. Thirdly, electron microscopy analysis showed a single AuNP entrapped per nanocarrier and no clusters of AuNPs were observed. Finally, the deep-red AuNHyb solutions exhibited a specific surface plasmon absorption peak at 525 nm, and a low 650 nm/530 nm ratio, which is characteristic for non-agglomerated AuNPs.49
3.5 Cellular uptake experiments
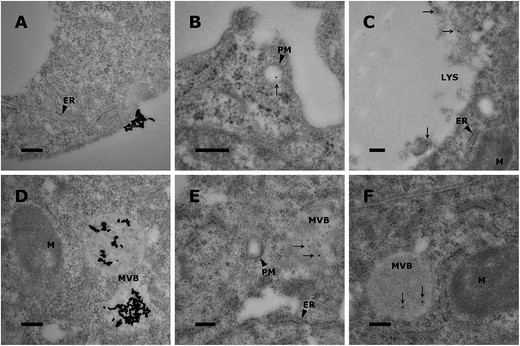
To demonstrate the use of AuNHybs for bioimaging, we performed uptake experiments with the AuNHybs in HepG2 cells exploiting the unique optical properties of AuNPs. The electron-density of AuNPs was used for further TEM analysis of cells (Fig. 5) as shown recently for surface-modified AuNPs.50 Due to the high quality and uniform size of AuNPs, TEM observations were possible without silver enhancement. Using this approach, fundamental insights about nanocarrier uptake and intracellular fate can be obtained. For example, proteins covering the surface of nanocarriers after administration to biological medium can influence cellular uptake. However, formation of a protein corona and opsonisation can be minimized using PEGylated nanocarriers (i.e. PEG–PCL nanoparticles or PEGylated liposomes).51,52 It remains to be elucidated to which degree protein adsorption onto non-modified nanocarriers such as conventional liposomes has to be taken into account for biomedical applications.53,54The interaction of free AuNPs with HepG2 cells is shown in Fig. 5A and D. Free AuNPs formed aggregates which were located on the cellular plasma membrane (Fig. 5A) or taken up into the cell (Fig. 5D). These observations are in agreement with published results.50,55 Fig. 5 shows the interaction with cells of lipid (panel B/E) or polymer based AuNHybs (panel C/F). In contrast to the uptake of free AuNPs, no AuNP aggregates were detected using AuNHybs. Different internalization steps were observed. An early stage in the cellular uptake mechanism was indicated by parts of the cellular plasma membrane around endosomes located near the cellular membrane (Fig. 5B). Compartments filled with more than one AuNP are most likely caused during maturation of different AuNHyb-filled vesicles (Fig. 5E/F).
AuNHybs could thus be used to examine nanoparticle–cell-interactions and the intracellular fate of the nanocarriers inside the cells using TEM, a technique which offers greater resolution compared to confocal microscopy. It should be noted that liposomes encapsulating AuNPs can act simultaneously as a carrier for fluorescent dyes (see ESI† for experimental details). For example rhodamine labelled phospholipids (Rho-PE) can be used to fluorescence label AuNHybs (Fig. S4b†). This offers the possibility to image fluorescent AuNHybs by confocal fluorescence microscopy or to quantify them with flow cytometry analysis.
4. Conclusions
In conclusion, we have developed a novel strategy for the preparation of lipid and polymer based AuNHybs. After encapsulation of the required reagents inside nanocarriers, formation of AuNPs was triggered by a temperature shift. In future, a similar approach could be used for the preparation of alternative metal nanohybrids such as silver nanoparticles. Different preparation techniques were used in combination with various nanomaterials. To the best of our knowledge, this nanoreactor approach is unique and was not used previously. The high reproducibility and versatility of our nanoreactor approach is unprecedented and makes this technology suitable for many nanomaterials. Microfluidics offers the possibility for an efficient and large scale production.The produced AuNHybs have a size of 70 nm to 130 nm, which makes them ideal for bioimaging applications.56 In addition, AuNHybs can be stored over a prolonged period of time (i.e. >3 months/4 °C) maintaining their initial size and monodispersity.
In order to target specific cells or tissues, AuNHybs can be easily modified using surface-conjugated receptor ligands.57 Our nanoreactor approach will be instrumental to develop a better understanding of cellular uptake and intracellular trafficking of targeted nanocarriers.
Acknowledgements
The authors declare no conflicting financial interest. Funding of the Stiftung zur Förderung des pharmazeutischen Nachwuchses in Basel is acknowledged. C. P.-B. was supported by the Swiss National Science Foundation (31003A-125423). D. W. thanks Lisa Stoltenburg for her support during this project. The authors thank Dr Adam Lister for editorial and Andrea Fehrenbach for graphical assistance. In addition, we thank Dr Susanne H. Schenk for her feedback on the manuscript, Dr Timothy Sharpe for his scientific input, and Prof. Henning Stahlberg (Director of C-CINA, Centre for Cellular Imaging and NanoAnalytics) and the Microscopy Center of the Biozentrum Basel for their support with electron microscopy techniques.Notes and references
- A. S. Thakor, J. Jokerst, C. Zavaleta, T. F. Massoud and S. S. Gambhir, Nano Lett., 2011, 11, 4029–4036 CrossRef CAS PubMed.
- D. F. Moyano, B. Duncan and V. M. Rotello, Methods Mol. Biol., 2013, 1025, 3–8 CAS.
- D. Kumar, N. Saini, N. Jain, R. Sareen and V. Pandit, Expert Opin. Drug Delivery, 2013, 10, 397–409 CrossRef CAS PubMed.
- R. Cao-Milán and L. M. Liz-Marzán, Expert Opin. Drug Delivery, 2014, 11, 741–752 CrossRef PubMed.
- L. C. Kennedy, L. R. Bickford, N. A. Lewinski, A. J. Coughlin, Y. Hu, E. S. Day, J. L. West and R. A. Drezek, Small, 2011, 7, 169–183 CrossRef CAS PubMed.
- E. Oh, K. Susumu, R. Goswami and H. Mattoussi, Langmuir, 2010, 26, 7604–7613 CrossRef CAS PubMed.
- C. Li, D. Li, G. Wan, J. Xu and W. Hou, Nanoscale Res. Lett., 2011, 6, 440 CrossRef PubMed.
- S. K. Sivaraman, S. Kumar and V. Santhanam, J. Colloid Interface Sci., 2011, 361, 543–547 CrossRef CAS PubMed.
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. Plech, J. Phys. Chem. B, 2006, 110, 15700–15707 CrossRef CAS PubMed.
- P. J. G. Goulet and R. B. Lennox, J. Am. Chem. Soc., 2010, 132, 9582–9584 CrossRef CAS PubMed.
- L. L. Lazarus, A. S.-J. Yang, S. Chu, R. L. Brutchey and N. Malmstadt, Lab Chip, 2010, 10, 3377–3379 RSC.
- W. Wang, Q.-Q. Wei, J. Wang, B.-C. Wang, S. Zhang and Z. Yuan, J. Colloid Interface Sci., 2013, 404, 223–229 CrossRef CAS PubMed.
- D. Pornpattananangkul, S. Olson, S. Aryal, M. Sartor, C.-M. Huang, K. Vecchio and L. Zhang, ACS Nano, 2010, 4, 1935–1942 CrossRef CAS PubMed.
- D. V. Volodkin, A. G. Skirtach and H. Möhwald, Angew. Chem., Int. Ed. Engl., 2009, 48, 1807–1809 CrossRef CAS PubMed.
- M. R. Preiss and G. D. Bothun, Expert Opin. Drug Delivery, 2011, 8, 1025–1040 CrossRef CAS PubMed.
- A. K. Rengan, M. Jagtap, A. de, R. Banerjee and R. Srivastava, Nanoscale, 2014, 6, 916–923 RSC.
- G. von White, Y. Chen, J. Roder-Hanna, G. D. Bothun and C. L. Kitchens, ACS Nano, 2012, 6, 4678–4685 CrossRef CAS PubMed.
- J. Nam, Y. S. Ha, S. Hwang, W. Lee, J. Song, J. Yoo and S. Kim, Nanoscale, 2013, 5, 10175–10178 RSC.
- H. Minematsu, T. Otani, T. Oohashi, M. Hirai, K. Oie, K. Igarashi and A. Ohtsuka, J. Electron Microsc., 2011, 60, 95–99 CrossRef PubMed.
- M. R. Rasch, E. Rossinyol, J. L. Hueso, B. W. Goodfellow, J. Arbiol and B. A. Korgel, Nano Lett., 2010, 10, 3733–3739 CrossRef CAS PubMed.
- S.-H. Park, S.-G. Oh, J.-Y. Mun and S.-S. Han, Colloids Surf., B, 2006, 48, 112–118 CrossRef CAS PubMed.
- L. Paasonen, T. Sipilä, A. Subrizi, P. Laurinmäki, S. J. Butcher, M. Rappolt, A. Yaghmur, A. Urtti and M. Yliperttula, J. Controlled Release, 2010, 147, 136–143 CrossRef CAS PubMed.
- C. Kojima, Y. Hirano, E. Yuba, A. Harada and K. Kono, Colloids Surf., B, 2008, 66, 246–252 CrossRef CAS PubMed.
- T. K. Sau, A. S. Urban, S. K. Dondapati, M. Fedoruk, M. R. Horton, A. L. Rogach, F. D. Stefani, J. O. Rädler and J. Feldmann, Colloids Surf., A, 2009, 342, 92–96 CrossRef CAS PubMed.
- R. Genç, G. Clergeaud, M. Ortiz and C. K. O'Sullivan, Langmuir, 2011, 27, 10894–10900 CrossRef PubMed.
- K. Hong, D. S. Friend, C. G. Glabe and D. Papahadjopoulos, Biochim. Biophys. Acta, 1983, 732, 320–323 CrossRef CAS.
- J. Huwyler, D. Wu and W. M. Pardridge, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 14164–14169 CrossRef CAS.
- T. Govender, S. Stolnik, M. C. Garnett, L. Illum and S. S. Davis, J. Controlled Release, 1999, 57, 171–185 CrossRef CAS.
- Y. Kim, B. Lee Chung, M. Ma, W. J. M. Mulder, Z. A. Fayad, O. C. Farokhzad and R. Langer, Nano Lett., 2012, 12, 3587–3591 CrossRef CAS PubMed.
- L. Y. Yeo, H.-C. Chang, P. P. Y. Chan and J. R. Friend, Small, 2011, 7, 12–48 CrossRef CAS PubMed.
- P. Detampel, D. Witzigmann, S. Krähenbühl and J. Huwyler, J. Drug Targeting, 2013, 22, 232–241 CrossRef PubMed.
- S. Deshpande and T. Pfohl, Biomicrofluidics, 2012, 6, 34120 CrossRef PubMed.
- M. E. Brennich, J.-F. Nolting, C. Dammann, B. Nöding, S. Bauch, H. Herrmann, T. Pfohl and S. Köster, Lab Chip, 2011, 11, 708–716 RSC.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- G. Frens, Nature (London), Phys. Sci., 1973, 241, 20–22 CrossRef CAS PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. Puntes, ACS Nano, 2010, 4, 3623–3632 CrossRef CAS PubMed.
- S. Mornet, O. Lambert, E. Duguet and A. Brisson, Nano Lett., 2005, 5, 281–285 CrossRef CAS PubMed.
- E. Jäger, A. Jäger, T. Etrych, F. C. Giacomelli, P. Chytil, A. Jigounov, J.-L. Putaux, B. Říhová, K. Ulbrich and P. Štěpánek, Soft Matter, 2012, 8, 9563–9575 RSC.
- I. V. Zhigaltsev, N. Belliveau, I. Hafez, A. K. K. Leung, J. Huft, C. Hansen and P. R. Cullis, Langmuir, 2012, 28, 3633–3640 CrossRef CAS PubMed.
- A. Jahn, W. N. Vreeland, M. Gaitan and L. E. Locascio, J. Am. Chem. Soc., 2004, 126, 2674–2675 CrossRef CAS PubMed.
- P. M. Valencia, P. A. Basto, L. Zhang, M. Rhee, R. Langer, O. C. Farokhzad and R. Karnik, ACS Nano, 2010, 4, 1671–1679 CrossRef CAS PubMed.
- N. Maurer, K. F. Wong, H. Stark, L. Louie, D. McIntosh, T. Wong, P. Scherrer, S. C. Semple and P. R. Cullis, Biophys. J., 2001, 80, 2310–2326 CrossRef CAS.
- C. Bonnaud, C. A. Monnier, D. Demurtas, C. Jud, D. Vanhecke, X. Montet, R. Hovius, M. Lattuada, B. Rothen-Rutishauser and A. Petri-Fink, ACS Nano, 2014, 8, 3451–3460 CrossRef CAS PubMed.
- U. Bilati, E. Allémann and E. Doelker, Eur. J. Pharm. Sci., 2005, 24, 67–75 CrossRef CAS PubMed.
- D. D. Lasic, B. Ceh, M. C. Stuart, L. Guo, P. M. Frederik and Y. Barenholz, Biochim. Biophys. Acta, 1995, 1239, 145–156 CrossRef.
- B. Ceh and D. D. Lasic, J. Colloid Interface Sci., 1997, 185, 9–18 CrossRef CAS.
- A. Fritze, F. Hens, A. Kimpfler, R. Schubert and R. Peschka-Süss, Biochim. Biophys. Acta, Biomembr., 2006, 1758, 1633–1640 CrossRef CAS PubMed.
- M. Camblin, P. Detampel, H. Kettiger, D. Wu, V. Balasubramanian and J. Huwyler, Int. J. Nanomed., 2014, 9, 2287–2298 Search PubMed.
- C.-C. Huang, Y.-F. Huang, Z. Cao, W. Tan and H.-T. Chang, Anal. Chem., 2005, 77, 5735–5741 CrossRef CAS PubMed.
- P. Nativo, I. A. Prior and M. Brust, ACS Nano, 2008, 2, 1639–1644 CrossRef CAS PubMed.
- D. Pozzi, V. Colapicchioni, G. Caracciolo, S. Piovesana, A. L. Capriotti, S. Palchetti, S. D. Grossi, A. Riccioli, H. Amenitsch and A. Laganà, Nanoscale, 2014, 6, 2782–2792 RSC.
- M. Lundqvist, J. Stigler, T. Cedervall, T. Berggård, M. B. Flanagan, I. Lynch, G. Elia and K. Dawson, ACS Nano, 2011, 5, 7503–7509 CrossRef CAS PubMed.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- M. Hadjidemetriou, Z. Al-Ahmady, M. Mazza, R. F. Collins, K. Dawson and K. Kostarelos, ACS Nano, 2015, 9, 8142–8156 CrossRef CAS PubMed.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef CAS PubMed.
- H. Kettiger, A. Schipanski, P. Wick and J. Huwyler, Int. J. Nanomed., 2013, 8, 3255–3269 Search PubMed.
- A. Wicki, D. Witzigmann, V. Balasubramanian and J. Huwyler, J. Controlled Release, 2015, 200, 138–157 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13967h |
| This journal is © The Royal Society of Chemistry 2015 |